Casein kinase 1 and disordered clock proteins form functionally equivalent, phospho-based circadian modules in fungi and mammals
- PMID: 35217617
- PMCID: PMC8892514
- DOI: 10.1073/pnas.2118286119
Casein kinase 1 and disordered clock proteins form functionally equivalent, phospho-based circadian modules in fungi and mammals
Abstract
Circadian clocks are timing systems that rhythmically adjust physiology and metabolism to the 24-h day-night cycle. Eukaryotic circadian clocks are based on transcriptional-translational feedback loops (TTFLs). Yet TTFL-core components such as Frequency (FRQ) in Neurospora and Periods (PERs) in animals are not conserved, leaving unclear how a 24-h period is measured on the molecular level. Here, we show that CK1 is sufficient to promote FRQ and mouse PER2 (mPER2) hyperphosphorylation on a circadian timescale by targeting a large number of low-affinity phosphorylation sites. Slow phosphorylation kinetics rely on site-specific recruitment of Casein Kinase 1 (CK1) and access of intrinsically disordered segments of FRQ or mPER2 to bound CK1 and on CK1 autoinhibition. Compromising CK1 activity and substrate binding affects the circadian clock in Neurospora and mammalian cells, respectively. We propose that CK1 and the clock proteins FRQ and PERs form functionally equivalent, phospho-based timing modules in the core of the circadian clocks of fungi and animals.
Keywords: CK1; FRQ; PER; circadian clock; intrinsically disordered.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
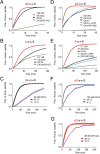
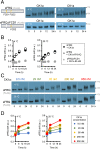
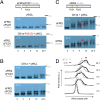

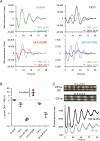

Comment in
-
Casein kinase rolls the dice in clocks from bread mold to humans.Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2201492119. doi: 10.1073/pnas.2201492119. Epub 2022 Mar 21. Proc Natl Acad Sci U S A. 2022. PMID: 35312371 Free PMC article. No abstract available.
Similar articles
-
Phosphorylation, disorder, and phase separation govern the behavior of Frequency in the fungal circadian clock.Elife. 2024 Mar 25;12:RP90259. doi: 10.7554/eLife.90259. Elife. 2024. PMID: 38526948 Free PMC article.
-
FRQ-CK1 Interaction Underlies Temperature Compensation of the Neurospora Circadian Clock.mBio. 2021 Jun 29;12(3):e0142521. doi: 10.1128/mBio.01425-21. Epub 2021 Jun 29. mBio. 2021. PMID: 34182774 Free PMC article.
-
FRQ-CK1 interaction determines the period of circadian rhythms in Neurospora.Nat Commun. 2019 Sep 25;10(1):4352. doi: 10.1038/s41467-019-12239-w. Nat Commun. 2019. PMID: 31554810 Free PMC article.
-
Phosphorylation Timers in the Neurospora crassa Circadian Clock.J Mol Biol. 2020 May 29;432(12):3449-3465. doi: 10.1016/j.jmb.2020.04.004. Epub 2020 Apr 17. J Mol Biol. 2020. PMID: 32305463 Review.
-
Posttranslational regulation of Neurospora circadian clock by CK1a-dependent phosphorylation.Cold Spring Harb Symp Quant Biol. 2007;72:177-83. doi: 10.1101/sqb.2007.72.025. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419275 Review.
Cited by
-
Functional analysis of 110 phosphorylation sites on the circadian clock protein FRQ identifies clusters determining period length and temperature compensation.G3 (Bethesda). 2023 Feb 9;13(2):jkac334. doi: 10.1093/g3journal/jkac334. G3 (Bethesda). 2023. PMID: 36537198 Free PMC article.
-
Exploration on cold adaptation of Antarctic lichen via detection of positive selection genes.IMA Fungus. 2024 Sep 9;15(1):29. doi: 10.1186/s43008-024-00160-x. IMA Fungus. 2024. PMID: 39252145 Free PMC article.
-
Antisense Transcription of the Neurospora Frequency Gene Is Rhythmically Regulated by CSP-1 Repressor but Dispensable for Clock Function.J Biol Rhythms. 2023 Jun;38(3):259-268. doi: 10.1177/07487304231153914. Epub 2023 Mar 1. J Biol Rhythms. 2023. PMID: 36876962 Free PMC article.
-
Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock.Biomedicines. 2025 Mar 13;13(3):705. doi: 10.3390/biomedicines13030705. Biomedicines. 2025. PMID: 40149680 Free PMC article. Review.
-
Are circadian amplitudes and periods correlated? A new twist in the story.F1000Res. 2024 Apr 23;12:1077. doi: 10.12688/f1000research.135533.2. eCollection 2023. F1000Res. 2024. PMID: 37771612 Free PMC article.
References
-
- Patke A., Young M. W., Axelrod S., Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020). - PubMed
-
- Diernfellner A. C. R., Brunner M., Phosphorylation timers in the Neurospora crassa circadian clock. J. Mol. Biol. 432, 3449–3465 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

