Kurarinone alleviated Parkinson's disease via stabilization of epoxyeicosatrienoic acids in animal model
- PMID: 35217618
- PMCID: PMC8892522
- DOI: 10.1073/pnas.2118818119
Kurarinone alleviated Parkinson's disease via stabilization of epoxyeicosatrienoic acids in animal model
Abstract
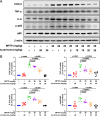
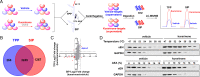
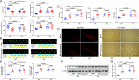
Parkinson's disease (PD) is one of the most common neurodegenerative disorders and is characterized by loss of dopaminergic neurons in the substantia nigra (SN), causing bradykinesia and rest tremors. Although the molecular mechanism of PD is still not fully understood, neuroinflammation has a key role in the damage of dopaminergic neurons. Herein, we found that kurarinone, a unique natural product from Sophora flavescens, alleviated the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced behavioral deficits and dopaminergic neurotoxicity, including the losses of neurotransmitters and tyrosine hydroxylase (TH)-positive cells (SN and striatum [STR]). Furthermore, kurarinone attenuated the MPTP-mediated neuroinflammation via suppressing the activation of microglia involved in the nuclear factor kappa B signaling pathway. The proteomics result of the solvent-induced protein precipitation and thermal proteome profiling suggest that the soluble epoxide hydrolase (sEH) enzyme, which is associated with the neuroinflammation of PD, is a promising target of kurarinone. This is supported by the increase of plasma epoxyeicosatrienoic acids (sEH substrates) and the decrease of dihydroxyeicosatrienoic acids (sEH products), and the results of in vitro inhibition kinetics, surface plasmon resonance, and cocrystallization of kurarinone with sEH revealed that this natural compound is an uncompetitive inhibitor. In addition, sEH knockout (KO) attenuated the progression of PD, and sEH KO plus kurarinone did not further reduce the protection of PD in MPTP-induced PD mice. These findings suggest that kurarinone could be a potential natural candidate for the treatment of PD, possibly through sEH inhibition.
Keywords: Parkinson’s disease; Sophora flavescens; kurarinone; soluble epoxide hydrolase.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Selikhova M., et al. , A clinico-pathological study of subtypes in Parkinson’s disease. Brain 132, 2947–2957 (2009). - PubMed
-
- Dickson D. W., et al. , Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 8, 1150–1157 (2009). - PubMed
-
- Grayson M., Parkinson’s disease. Nature 538, S1 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

