Structure-guided engineering of tick evasins for targeting chemokines in inflammatory diseases
- PMID: 35217625
- PMCID: PMC8892493
- DOI: 10.1073/pnas.2122105119
Structure-guided engineering of tick evasins for targeting chemokines in inflammatory diseases
Abstract
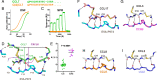
As natural chemokine inhibitors, evasin proteins produced in tick saliva are potential therapeutic agents for numerous inflammatory diseases. Engineering evasins to block the desired chemokines and avoid off-target side effects requires structural understanding of their target selectivity. Structures of the class A evasin EVA-P974 bound to human CC chemokine ligands 7 and 17 (CCL7 and CCL17) and to a CCL8-CCL7 chimera reveal that the specificity of class A evasins for chemokines of the CC subfamily is defined by conserved, rigid backbone-backbone interactions, whereas the preference for a subset of CC chemokines is controlled by side-chain interactions at four hotspots in flexible structural elements. Hotspot mutations alter target preference, enabling inhibition of selected chemokines. The structure of an engineered EVA-P974 bound to CCL2 reveals an underlying molecular mechanism of EVA-P974 target preference. These results provide a structure-based framework for engineering evasins as targeted antiinflammatory therapeutics.
Keywords: chemokines; inflammatory diseases; protein engineering; tick evasins.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
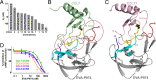


Similar articles
-
Engineering broad-spectrum inhibitors of inflammatory chemokines from subclass A3 tick evasins.Nat Commun. 2023 Jul 14;14(1):4204. doi: 10.1038/s41467-023-39879-3. Nat Commun. 2023. PMID: 37452046 Free PMC article.
-
Structural basis of chemokine recognition by the class A3 tick evasin EVA-ACA1001.Protein Sci. 2024 Jun;33(6):e4999. doi: 10.1002/pro.4999. Protein Sci. 2024. PMID: 38723106 Free PMC article.
-
The N-terminal domain of a tick evasin is critical for chemokine binding and neutralization and confers specific binding activity to other evasins.J Biol Chem. 2018 Apr 20;293(16):6134-6146. doi: 10.1074/jbc.RA117.000487. Epub 2018 Feb 27. J Biol Chem. 2018. PMID: 29487134 Free PMC article.
-
Evasins: Tick Salivary Proteins that Inhibit Mammalian Chemokines.Trends Biochem Sci. 2020 Feb;45(2):108-122. doi: 10.1016/j.tibs.2019.10.003. Epub 2019 Nov 1. Trends Biochem Sci. 2020. PMID: 31679840 Free PMC article. Review.
-
Using evasins to target the chemokine network in inflammation.Adv Protein Chem Struct Biol. 2020;119:1-38. doi: 10.1016/bs.apcsb.2019.09.003. Epub 2019 Nov 26. Adv Protein Chem Struct Biol. 2020. PMID: 31997766 Review.
Cited by
-
Discovery and pharmacophoric characterization of chemokine network inhibitors using phage-display, saturation mutagenesis and computational modelling.Nat Commun. 2023 Sep 16;14(1):5763. doi: 10.1038/s41467-023-41488-z. Nat Commun. 2023. PMID: 37717048 Free PMC article.
-
Engineering broad-spectrum inhibitors of inflammatory chemokines from subclass A3 tick evasins.Nat Commun. 2023 Jul 14;14(1):4204. doi: 10.1038/s41467-023-39879-3. Nat Commun. 2023. PMID: 37452046 Free PMC article.
-
Rational Design of High Affinity Interaction Between CC Chemokine Binding Protein vCCI and CCL17/TARC.Biochemistry. 2024 Sep 17;63(18):2235-2239. doi: 10.1021/acs.biochem.4c00298. Epub 2024 Aug 28. Biochemistry. 2024. PMID: 39194151 Free PMC article.
-
Structural basis of chemokine recognition by the class A3 tick evasin EVA-ACA1001.Protein Sci. 2024 Jun;33(6):e4999. doi: 10.1002/pro.4999. Protein Sci. 2024. PMID: 38723106 Free PMC article.
References
-
- Zlotnik A., Yoshie O., Chemokines: A new classification system and their role in immunity. Immunity 12, 121–127 (2000). - PubMed
-
- Moore J. P., et al. , M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H906–H917 (2015). - PubMed
-
- Zhao S., Wu B., Stevens R. C., Advancing chemokine GPCR structure based drug discovery. Structure 27, 405–408 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

