Spinal cord injury reprograms muscle fibroadipogenic progenitors to form heterotopic bones within muscles
- PMID: 35217633
- PMCID: PMC8881504
- DOI: 10.1038/s41413-022-00188-y
Spinal cord injury reprograms muscle fibroadipogenic progenitors to form heterotopic bones within muscles
Abstract
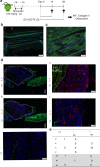
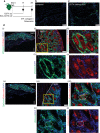
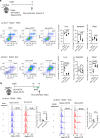
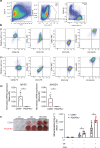
The cells of origin of neurogenic heterotopic ossifications (NHOs), which develop frequently in the periarticular muscles following spinal cord injuries (SCIs) and traumatic brain injuries, remain unclear because skeletal muscle harbors two progenitor cell populations: satellite cells (SCs), which are myogenic, and fibroadipogenic progenitors (FAPs), which are mesenchymal. Lineage-tracing experiments using the Cre recombinase/LoxP system were performed in two mouse strains with the fluorescent protein ZsGreen specifically expressed in either SCs or FAPs in skeletal muscles under the control of the Pax7 or Prrx1 gene promoter, respectively. These experiments demonstrate that following muscle injury, SCI causes the upregulation of PDGFRα expression on FAPs but not SCs and the failure of SCs to regenerate myofibers in the injured muscle, with reduced apoptosis and continued proliferation of muscle resident FAPs enabling their osteogenic differentiation into NHOs. No cells expressing ZsGreen under the Prrx1 promoter were detected in the blood after injury, suggesting that the cells of origin of NHOs are locally derived from the injured muscle. We validated these findings using human NHO biopsies. PDGFRα+ mesenchymal cells isolated from the muscle surrounding NHO biopsies could develop ectopic human bones when transplanted into immunocompromised mice, whereas CD56+ myogenic cells had a much lower potential. Therefore, NHO is a pathology of the injured muscle in which SCI reprograms FAPs to undergo uncontrolled proliferation and differentiation into osteoblasts.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Wittenberg, R. H., Peschke, U. & Botel, U. Heterotopic ossification after spinal cord injury. Epidemiology and risk factors. J. Bone Joint Surg. Br.74, 215–218 (1992). - PubMed
-
- Reznik JE, et al. Prevalence and risk-factors of neurogenic heterotopic ossification in traumatic spinal cord and traumatic brain injured patients admitted to specialised units in Australia. J. Musculoskelet. Neuronal Interact. 2014;14:19–28. - PubMed
-
- van Kuijk, A. A., Geurts, A. C. & van Kuppevelt, H. J. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord.40, 313–326 (2002). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

