Increased retention of tau PET ligand [18F]-AV1451 in Alzheimer's Disease Psychosis
- PMID: 35217635
- PMCID: PMC8881582
- DOI: 10.1038/s41398-022-01850-z
Increased retention of tau PET ligand [18F]-AV1451 in Alzheimer's Disease Psychosis
Abstract
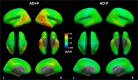
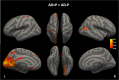
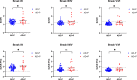
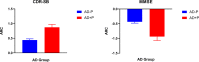
Psychosis in Alzheimer's disease (AD) represents a distinct disease subtype with a more rapid progression of illness evidenced by an increased velocity of cognitive decline and a hastened mortality. Previous biomarker and post-mortem studies have implicated tau neuropathology as a possible mediator of the accelerated decline in AD psychosis. Tau positron emission tomography (PET) neuroimaging provides the opportunity to evaluate tau pathology in-vivo, so that clinical symptomatology can be correlated with disease pathology. [18F]-AV1451 (Flortaucipir) is a PET ligand with high affinity for insoluble paired-helical filaments (PHFs) of hyperphosphorylated tau. In order to determine whether the development of psychosis and worsened prognosis in AD is associated with an increased burden of tau pathology that can be identified with tau imaging, we identified subjects within the Alzheimer's disease neuroimaging initiative (ADNI) who had [18F]-AV1451 imaging at baseline and became psychotic over the course of the study (N = 17) and matched them 1:3 for gender, age, and education to subjects who had [18F]-AV1451 imaging at baseline and did not become psychotic (N = 50). We compared baseline [18F]-AV1451 retention, in addition to cognitive and functional baseline and longitudinal change, in those who became psychotic over the course of participation in ADNI with those who did not. Results suggest that increases in tau pathology in frontal, medial temporal, and occipital cortices, visualized with [18F]-AV1451 binding, are associated with psychosis and a more rapid cognitive and functional decline.
© 2022. The Author(s).
Conflict of interest statement
JJG has received grant support from the Alzheimer’s Association (AACFD-16-438886) paid to institution. JH is a member of the Communications and Social Media Committee of the New York State Association of Neuropsychology (volunteer position). MLG has received support (paid to the institution) from AbbVie, Eisai, Janssen, and National Institute on Aging (NIA); MLG has received personal support from METiS Pharmaceuticals; MLG has participated in the advisory board of Eisai. JK has received support from NIA, AFA, and Acadia pharma, all paid to the institution. The rest of the authors have nothing to disclose.
Figures
References
-
- Sweet RA, Nimgaonkar VL, Devlin B, Jeste DV. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol Psychiatry. 2003;8:383–92. - PubMed
-
- Jeste DV, Wragg RE, Salmon DP, Harris MJ, Thal LJ. Cognitive deficits of patients with Alzheimer’s disease with and without delusions. Am J Psychiatry. 1992;149:184–9. - PubMed
-
- Gilley DW, Wilson RS, Bennett DA, Bernard BA, Fox JH. Predictors of behavioral disturbance in Alzheimer’s disease. J Gerontol. 1991;46:P362–71. - PubMed
-
- Vilalta-Franch J, Lopez-Pousa S, Calvo-Perxas L, Garre-Olmo J. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21:1135–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

