Increased fMRI connectivity upon chemogenetic inhibition of the mouse prefrontal cortex
- PMID: 35217677
- PMCID: PMC8881459
- DOI: 10.1038/s41467-022-28591-3
Increased fMRI connectivity upon chemogenetic inhibition of the mouse prefrontal cortex
Abstract
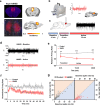
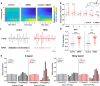
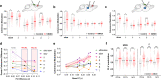
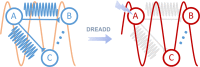
While shaped and constrained by axonal connections, fMRI-based functional connectivity reorganizes in response to varying interareal input or pathological perturbations. However, the causal contribution of regional brain activity to whole-brain fMRI network organization remains unclear. Here we combine neural manipulations, resting-state fMRI and in vivo electrophysiology to probe how inactivation of a cortical node causally affects brain-wide fMRI coupling in the mouse. We find that chronic inhibition of the medial prefrontal cortex (PFC) via overexpression of a potassium channel increases fMRI connectivity between the inhibited area and its direct thalamo-cortical targets. Acute chemogenetic inhibition of the PFC produces analogous patterns of fMRI overconnectivity. Using in vivo electrophysiology, we find that chemogenetic inhibition of the PFC enhances low frequency (0.1-4 Hz) oscillatory power via suppression of neural firing not phase-locked to slow rhythms, resulting in increased slow and δ band coherence between areas that exhibit fMRI overconnectivity. These results provide causal evidence that cortical inactivation can counterintuitively increase fMRI connectivity via enhanced, less-localized slow oscillatory processes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

