Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay
- PMID: 35217718
- PMCID: PMC8881501
- DOI: 10.1038/s42003-022-03090-9
Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay
Abstract
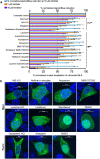
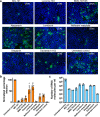
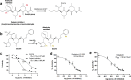
SARS-CoV-2 proteases Mpro and PLpro are promising targets for antiviral drug development. In this study, we present an antiviral screening strategy involving a novel in-cell protease assay, antiviral and biochemical activity assessments, as well as structural determinations for rapid identification of protease inhibitors with low cytotoxicity. We identified eight compounds with anti-SARS-CoV-2 activity from a library of 64 repurposed drugs and modeled at protease active sites by in silico docking. We demonstrate that Sitagliptin and Daclatasvir inhibit PLpro, and MG-101, Lycorine HCl, and Nelfinavir mesylate inhibit Mpro of SARS-CoV-2. The X-ray crystal structure of Mpro in complex with MG-101 shows a covalent bond formation between the inhibitor and the active site Cys145 residue indicating its mechanism of inhibition is by blocking the substrate binding at the active site. Thus, we provide methods for rapid and effective screening and development of inhibitors for blocking virus polyprotein processing as SARS-CoV-2 antivirals. Additionally, we show that the combined inhibition of Mpro and PLpro is more effective in inhibiting SARS-CoV-2 and the delta variant.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

