Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome
- PMID: 35217818
- PMCID: PMC9525652
- DOI: 10.1038/s41401-022-00876-9
Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome
Abstract
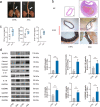
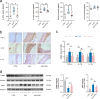
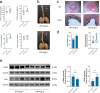
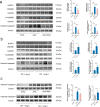
Abdominal aortic aneurysm (AAA) is defined as a dilated aorta in diameter at least 1.5 times of a normal aorta. Our previous studies found that activating α7 nicotinic acetylcholine receptor (α7nAChR) had a protective effect on vascular injury. This work was to investigate whether activating α7nAChR could influence AAA formation and explore its mechanisms. AAA models were established by angiotensin II (Ang II) infusion in ApoE-/- mice or in wild type and α7nAChR-/- mice. In vitro mouse aortic smooth muscle (MOVAS) cells were treated with tumor necrosis factor-α (TNF-α). PNU-282987 was chosen to activate α7nAChR. We found that cell pyroptosis effector GSDMD and NLRP3 inflammasome were activated in abdominal aorta, and inflammatory cytokines in serum were elevated in AAA models of ApoE-/- mice. Activating α7nAChR reduced maximal aortic diameters, preserved elastin integrity and decreased inflammatory responses in ApoE-/- mice with Ang II infusion. While α7nAChR-/- mice led to aggravated aortic injury and increased inflammatory cytokines with Ang II infusion when compared with wild type. Moreover, activating α7nAChR inhibited NLRP3/caspase-1/GSDMD pathway in AAA model of ApoE-/- mice, while α7nAChR deficiency promoted this pathway. In vitro, N-acetylcysteine (NAC) inhibited NLRP3 inflammasome activation and NLRP3 knockdown reduced GSDMD expression, in MOVAS cells treated with TNF-α. Furthermore, activating α7nAChR inhibited oxidative stress, reduced NLRP3/GSDMD expression, and decreased cell pyroptosis in MOVAS cells with TNF-α. In conclusion, our study found that activating α7nAChR retarded AAA through inhibiting pyroptosis mediated by NLRP3 inflammasome. These suggested that α7nAChR would be a potential pharmacological target for AAA.
Keywords: NLRP3 inflammasome; abdominal aortic aneurysm; cell pyroptosis; inflammation; α7nAChR.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE, et al. Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart. 2014;9:171–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

