Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors
- PMID: 35217892
- PMCID: PMC9411268
- DOI: 10.1007/s00262-022-03169-6
Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors
Abstract
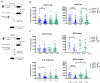
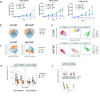
Human gut microbial species found to associate with clinical responses to immune checkpoint inhibitors (ICIs) are often tested in mice using fecal microbiota transfer (FMT), wherein tumor responses in recipient mice may recapitulate human responses to ICI treatment. However, many FMT studies have reported only limited methodological description, details of murine cohorts, and statistical methods. To investigate the reproducibility and robustness of gut microbial species that impact ICI responses, we performed human to germ-free mouse FMT using fecal samples from patients with non-small cell lung cancer who had a pathological response or nonresponse after neoadjuvant ICI treatment. R-FMT mice yielded greater anti-tumor responses in combination with anti-PD-L1 treatment compared to NR-FMT, although the magnitude varied depending on mouse cell line, sex, and individual experiment. Detailed investigation of post-FMT mouse microbiota using 16S rRNA amplicon sequencing, with models to classify and correct for biological variables, revealed a shared presence of the most highly abundant taxa between the human inocula and mice, though low abundance human taxa colonized mice more variably after FMT. Multiple Clostridium species also correlated with tumor outcome in individual anti-PD-L1-treated R-FMT mice. RNAseq analysis revealed differential expression of T and NK cell-related pathways in responding tumors, irrespective of FMT source, with enrichment of these cell types confirmed by immunohistochemistry. This study identifies several human gut microbial species that may play a role in clinical responses to ICIs and suggests attention to biological variables is needed to improve reproducibility and limit variability across experimental murine cohorts.
Keywords: Biological variables; Fecal microbiota transfer; Gut microbiome; Immune checkpoint inhibitors; Lung cancer; Neoadjuvant.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
JRW reports equity ownership of Resphera Biosciences. JEC reports consultant fees for AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Flame Biosciences, Novartis, Regeneron, Guardant Health, and Jansen. JN reports research grants from Merck and AstraZeneca; consulting for AstraZeneca, Bristol-Myers Squibb, Takeda, Pfizer, Daiichi Sankyo, and Roche/Genentech; and honoraria from AstraZeneca and Bristol-Myers Squibb. PMF reports research grants from AstraZeneca, Bristol-Myers Squibb, Corvus, and Novartis. PMF reports consulting for Amgen, AstraZeneca, Bristol-Myers Squibb, Daiichi, Janssen, and Iteos. PMF reports serving as data safety monitoring board member for Flame Biosciences and Polaris. JEC reports consultant fees from AstraZeneca, Flame Biosciences, Genentech, Merck, Novartis, Jannsen. JDS has received consulting fees and honoraria from BMS, Merck, Roche, Amgen, AstraZeneca and Protalix Biotherapeutics; and he has received research grants from AstraZeneca, Merck, Roche and CLS therapeutics. JER has received advising/consulting fees from Oncocyte. DMP reports research support from AstraZeneca, Bristol Myers Squibb, and Compugen. DMP reports consulting for Aduro Biotech, Amgen, Astra Zeneca, Astellas, Bayer, Camden Partners, Compugen, DNAtrix, Dracen, Dynavax, Ervaxx, Five Prime Therapeutics, RAPT Therapeutics, Immunomic Therapeutics, Immunocore, Janssen, Merck, Potenza, Rock Springs Capital, Tizona, Trieza Therapeutics, Vaccitech, WindMil. DMP reports stock/ownership in Aduro Biotech, Dracen, Ervaxx, Five Prime Therapeutics, Tizona, Trieza Therapeutics, and WindMil. CLS reports research grants from Bristol-Myers Squibb and Janssen and personal fees from Ferring, outside the submitted work.
Figures





References
-
- Brahmer J, Horn L, Jackman D, et al (2017) Abstract CT077: Five-year follow-up from the CA209–003 study of nivolumab in previously treated advanced non-small cell lung cancer (NSCLC): Clinical characteristics of long-term survivors. In: Cancer Research. American Association for Cancer Research (AACR), pp CT077–CT077
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

