Viral hepatitis and the cascade of care among people living with HIV in the Asia-Pacific
- PMID: 35218151
- PMCID: PMC9402797
- DOI: 10.1111/hiv.13280
Viral hepatitis and the cascade of care among people living with HIV in the Asia-Pacific
Abstract
Background: Although the prevalence and mortality of hepatitis is high in the Asia-Pacific region, few studies are available on the diagnosis, treatment, and cure rates for viral hepatitis among people living with HIV in this area. This study aims to report the cascade of care (CoC) for hepatitis B (HBV) and C (HCV) among people living with HIV receiving combined antiretroviral therapy (ART).
Methods: Patients enrolled in the TREAT Asia HIV Observational Database Low Intensity Transfer (TAHOD-LITE) cohort, on ART, and with follow-up data from 2010 to 2019 were included. Patients were determined as positive for HCV or HBV co-infection if they ever tested positive for HCV antibody (anti-HCV) or HBV surface antigen (HBsAg), respectively.
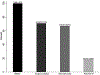
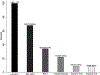
Results: In total, 39% (8612/22 340) of the adult HIV cohort had undergone HBsAg testing, with 8% (672/8612) testing positive. HBV CoC demonstrated that 71% (474/672) of those with HBsAg positive results initiated treatment, 67% (318/474) of those on treatment had HBV DNA testing to evaluate treatment progression, and 18% (58/318) of those tested reached viral suppression. Of the cohort, 37% (8231/22 340) had anti-HCV testing, of whom 10% (779/8231) tested positive. The HCV CoC showed that 68% (526/779) of those with positive anti-HCV tests had HCV RNA tests, of whom 51% (267/526) had detectable HCV RNA. Among those with detectable HCV RNA, 65% (174/267) initiated HCV treatment. Of the 40% (69/174) who initiated HCV treatment, 90% (62/69) reached sustained virological response.
Conclusion: Our findings identified less frequent testing in the healthcare system and limited access to treatment as gaps in the CoC for viral hepatitis. More routine HCV RNA and HBV DNA testing is required for patients with positive screening tests to identify those in need of treatment.
Keywords: Asia-Pacific; HBV; HCV; PLHIV; cascade of care; viral hepatitis.
© 2022 British HIV Association.
Conflict of interest statement
Conflict of Interest statement
The authors have no conflict of interest to disclose.
Figures
References
-
- WorldHealthOrganization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: 2015. - PubMed
-
- WorldHealthOrganization(WHO). Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. Geneva: World Health Organization; 2018. - PubMed
-
- WorldHealthOrganization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. In: HIV/AIDS Do, editor. Geneva, Switzerland: WHO Document Production Services; 2016.
-
- WorldHealthOrganization. Monitoring and evaluation for viral hepatitis B and C: recommended indicators and framework. Geneva, Switzerland: World Health Organization; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical