Unprecedented Mushroom Polyketide Synthases Produce the Universal Anthraquinone Precursor
- PMID: 35218274
- PMCID: PMC9325552
- DOI: 10.1002/anie.202116142
Unprecedented Mushroom Polyketide Synthases Produce the Universal Anthraquinone Precursor
Abstract
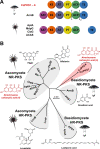
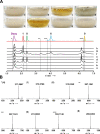
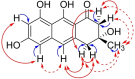
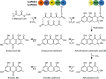
(Pre-)anthraquinones are widely distributed natural compounds and occur in plants, fungi, microorganisms, and animals, with atrochrysone (1) as the key biosynthetic precursor. Chemical analyses established mushrooms of the genus Cortinarius-the webcaps-as producers of atrochrysone-derived octaketide pigments. However, more recent genomic data did not provide any evidence for known atrochrysone carboxylic acid (4) synthases nor any other polyketide synthase (PKS) producing oligocyclic metabolites. Here, we describe an unprecedented class of non-reducing (NR-)PKS. In vitro assays with recombinant enzyme in combination with in vivo product formation in the heterologous host Aspergillus niger established CoPKS1 and CoPKS4 of C. odorifer as members of a new class of atrochrysone carboxylic acid synthases. CoPKS4 catalyzed both hepta- and octaketide synthesis and yielded 6-hydroxymusizin (6), along with 4. These first mushroom PKSs for oligocyclic products illustrate how the biosynthesis of bioactive natural metabolites evolved independently in various groups of life.
Keywords: Anthraquinones; Biosynthesis; Fungi; Octaketides; Polyketide Synthase.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Diaz-Muñoz G., Miranda I. L., Sartori S. K., de Rezende D. C., Diaz M. A. N., Stud. Nat. Prod. Chem. 2018, 58, 313–338.
-
- None
-
- Takido M., Takahashi S., Masuda K., Yasukawa K., Lloydia 1977, 40, 191–194;
-
- Gill M., Steglich W. in Progress in the Chemistry of Organic Natural Products, Vol. 51 (Eds.: Herz W., Grisebach H., Kirby G. W., Tamm C.), Springer, Heidelberg, 1987, pp. 125–174.
-
- Hüttel W., Müller M., Nat. Prod. Rep. 2021, 38, 1011–1043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

