A short HLA-DRA isoform binds the HLA-DR2 heterodimer on the outer domain of the peptide-binding site
- PMID: 35218721
- PMCID: PMC9007275
- DOI: 10.1016/j.abb.2022.109156
A short HLA-DRA isoform binds the HLA-DR2 heterodimer on the outer domain of the peptide-binding site
Abstract
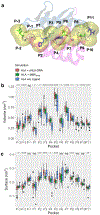
The human leukocyte antigen (HLA) locus encodes a large group of proteins governing adaptive and innate immune responses. Among them, HLA class II proteins form α/β heterodimers on the membrane of professional antigen-presenting cells (APCs), where they display both, self and pathogen-derived exogenous antigens to CD4+ T lymphocytes. We have previously shown that a shorter HLA-DRA isoform (sHLA-DRA) lacking 25 amino acids can be presented onto the cell membrane via binding to canonical HLA-DR2 heterodimers. Here, we employed atomistic molecular dynamics simulations to decipher the binding position of sHLA-DRA and its structural impact on functional regions of the HLA-DR2 molecule. We show that a loop region exposed only in the short isoform (residues R69 to G83) is responsible for binding to the outer domain of the HLA-DR2 peptide-binding site, and experimentally validated the critical role of F76 in mediating such interaction. Additionally, sHLA-DRA allosterically modifies the peptide-binding pocket conformation. In summary, this study unravels key molecular mechanisms underlying sHLA-DRA function, providing important insights into the role of full-length proteins in structural modulation of HLA class II receptors.
Keywords: Antigen presentation; Molecular dynamics; Protein-protein binding; Structural modulation.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no competing interests. All the data supporting the findings of this study are available from the corresponding author upon reasonable request.
Figures


References
-
- Kulski JK, Shiina T, Anzai T, Kohara S, Inoko H, Comparative genomic analysis of the MHC: the evolution of class I duplication blocks, diversity and complexity from shark to man, Immunol. Rev 190 (2002) 95–122. - PubMed
-
- Neefjes J, Jongsma MLM, Paul P, Bakke O, Towards a systems understanding of MHC class I and MHC class II antigen presentation, Nat. Rev. Immunol 11 (2011) 823–836. - PubMed
-
- McCluskey J, Peh C, The human leucocyte antigens and clinical medicine: an overview, Rev Immunogenet. 1 (1999) 3–20. - PubMed
-
- Stern LJ, Brown JH, Jardetzky TS, Gorgat JC, Urban RG, Strominger JL, Wiley DC, Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide, Nature. 368 (1994) 215–221. - PubMed
-
- Madden DR, Gorga JC, Strominger JL, Wiley DC, The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC, Cell. 70 (1992) 1035–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

