Global challenges in preparedness and response to epidemic infectious diseases
- PMID: 35218930
- PMCID: PMC8864962
- DOI: 10.1016/j.ymthe.2022.02.022
Global challenges in preparedness and response to epidemic infectious diseases
Abstract
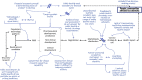
Lessons drawn from successes and failures with coronavirus disease 2019 (COVID-19) and Ebola virus disease (EVD) should help shaping a robust health innovation system for infectious disease epidemics. Epidemic response research and development (R&D) can be mobilized quickly for public health priorities and yield medicinal products within months. However, to resolve epidemics, technological advances must be equitably accessible and deployed, and these examples expose the limitations of a supply-driven, fragmented R&D ecosystem relying primarily on the private sector to deliver health products. Efficient epidemic response requires a coordinated public health-focused, end-to-end R&D ecosystem for the development, registration, availability, and use of pharmaceutical products. Because pivotal clinical trials can only be conducted during outbreaks, significant preparation must be done beforehand: strengthening clinical research capacity and developing pre-positioned trial protocols and clinical characterization protocols, as well as conducting discovery and pre-clinical research, manufacturing, and early clinical testing of candidate products. This will allow for speedy execution of clinical research early into an outbreak and delivering products within a short time. Effective interventions should be adopted and deployed ensuring equitable access during the ongoing outbreak. Measures to make products available where and when needed must be integrated throughout the R&D value chain.
Keywords: COVID-19; Ebola virus disease; health technology; infectious disease epidemic; innovation; medicinal products; preparedness; response.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest Both authors declare no conflict of interest.
Figures


References
-
- US Department of Health and Human Services . 3rd edition. 2006. Principles of Epidemiology in Public Health Practice.https://www.cdc.gov/csels/dsepd/ss1978/SS1978.pdf
-
- González-Muniesa P., Mártinez-González M.A., Hu F., Deprés J.P., Matsuzawa Y., Loos R.J.F., Moreno L.A., Bray G.A., Martinez J.A. Obesity. Nat. Rev. Dis. Primers. 2017;3:17034. - PubMed
-
- Skolnick P. The opioid epidemic: crisis and solutions. Annu. Rev. Pharmacol. Toxicol. 2018;58:143–159. - PubMed
-
- United Nations . 2015. 17 Sustainable Development Goals.https://sdgs.un.org/goals
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

