Inhibition of MEK-ERK signaling reduces seizures in two mouse models of tuberous sclerosis complex
- PMID: 35219048
- PMCID: PMC8930622
- DOI: 10.1016/j.eplepsyres.2022.106890
Inhibition of MEK-ERK signaling reduces seizures in two mouse models of tuberous sclerosis complex
Abstract
Tuberous sclerosis complex (TSC) is a monogenic disorder characterized by hyperactivation of the mTOR signaling pathway and developmental brain malformations leading to intractable epilepsy. Although treatment with the recently approved mTOR inhibitor, everolimus, results in clinically relevant seizure suppression in up to 40% of TSC patients, seizures remain uncontrolled in a large number of cases, underscoring the need to identify novel treatment targets. The MEK-ERK signaling pathway has been found to be aberrantly activated in TSC and inhibition of MEK-ERK activity independently of mTOR rescued neuronal dendrite overgrowth in mice modeling TSC neuropathology. Here, we evaluated the efficacy of MEK-ERK inhibition on seizures in two mouse models of TSC. We found that treatment with the MEK inhibitor PD0325901 (mirdametinib) significantly reduced seizure activity in both TSC mouse models. These findings support inhibiting MEK-ERK activity as a potential alternative strategy to treat seizures in TSC.
Keywords: Epilepsy; MAPK; MEK inhibitor; MEK-ERK signaling; Seizures; Tuberous sclerosis complex.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
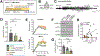

References
-
- Osborne JP, Fryer A, Webb D, Epidemiology of tuberous sclerosis. Ann N Y Acad Sci 615, 125–127 (1991). - PubMed
-
- Crino PB, Nathanson KL, Henske EP, The tuberous sclerosis complex. N Engl J Med 355, 1345–1356 (2006). - PubMed
-
- Kwiatkowski DJ, Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet 67, 87–96 (2003). - PubMed
-
- Curatolo P, Jozwiak S, Nabbout R, T. S. C. C. M. f. SEGA, Epilepsy M, Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol 16, 582–586 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

