Quantitation of the next-generation imipridone ONC206 in human plasma by a simple and sensitive UPLC-MS/MS assay for clinical pharmacokinetic application
- PMID: 35219065
- PMCID: PMC8983588
- DOI: 10.1016/j.jpba.2022.114685
Quantitation of the next-generation imipridone ONC206 in human plasma by a simple and sensitive UPLC-MS/MS assay for clinical pharmacokinetic application
Abstract
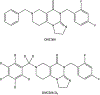
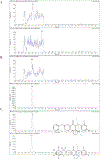
ONC206 is an imipridone derivative that is being developed clinically as a single agent given orally in a first-in-human trial (NCT04541082). This ongoing clinical trial requires pharmacokinetic analysis of ONC206 to fully characterize its pharmacologic profile. There is currently no published bioanalytical method for ONC206 quantitation. To understand the clinical pharmacokinetics of ONC206, a sensitive yet simple uHPLC-MS/MS method for quantitation of ONC206 in human plasma was developed. Protein-precipitation allowed rapid and sensitive bioanalytical measurement of ONC206 in human plasma. A Phenomenex Kinetex C18 (50 ×2.1 mm, 1.3 µm, 100 Å) analytical column achieved symmetrical and sharp chromatography peaks of ONC206 and the internal standard, [2H]7-ONC206, which were detected using multiple reaction monitoring. The assay calibration range was 1-500 ng/mL and was best fit by a linear regression model (r2 > 0.99732 ± 0.0010). The method proved accurate (< ± 9% deviation), precise (<11%CV), selective and specific with no interference and low inter-lot matrix variability. ONC206 demonstrated excellent short-term, long-term, and multiple freeze-thaw cycle stability in solution and human plasma. This fully validated method was used to quantitate ONC206 plasma concentrations from patients enrolled in the aforementioned clinical trial at the NCI to demonstrate its clinical applicability.
Keywords: Clinical pharmacology; Mass spectrometry; ONC206; Ultra-HPLC.
Published by Elsevier B.V.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

