Azadirachta indica A. Juss bark extract and its Nimbin isomers restrict β-coronaviral infection and replication
- PMID: 35219218
- PMCID: PMC8844965
- DOI: 10.1016/j.virol.2022.01.002
Azadirachta indica A. Juss bark extract and its Nimbin isomers restrict β-coronaviral infection and replication
Abstract
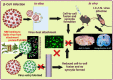
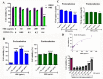
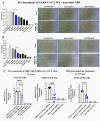
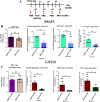
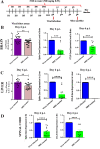
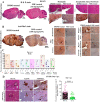
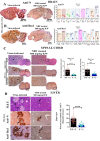
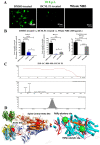
Emerging mutations in the SARS-CoV-2 genome pose a challenge for vaccine development and antiviral therapy. The antiviral efficacy of Azadirachta indica bark extract (NBE) was assessed against SARS-CoV-2 and m-CoV-RSA59 infection. Effects of in vivo intranasal or oral NBE administration on viral load, inflammatory response, and histopathological changes were assessed in m-CoV-RSA59-infection. NBE administered inhibits SARS-CoV-2 and m-CoV-RSA59 infection and replication in vitro, reducing Envelope and Nucleocapsid gene expression. NBE ameliorates neuroinflammation and hepatitis in vivo by restricting viral replication and spread. Isolated fractions of NBE enriched in Nimbin isomers shows potent inhibition of m-CoV-RSA59 infection in vitro. In silico studies revealed that NBE could target Spike and RdRp of m-CoV and SARS-CoV-2 with high affinity. NBE has a triterpenoids origin that may allow them to competitively target panoply of viral proteins to inhibit mouse and different strains of human coronavirus infections, suggesting its potential as an antiviral against pan-β-Coronaviruses.
Keywords: Antiviral; Azadirachtaindica A. Juss (Neem bark extract); Beta-coronavirus; Epinimbin/Nimbin; Inhibitor of viral entry and spread; RdRp (RNA dependant RNA polymerase); SARS-CoV-2; Virus spike protein; m-CoV-MHV-A59/RSA59.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures
References
-
- Appelberg S., Gupta S., Svensson Akusjarvi S., Ambikan A.T., Mikaeloff F., Saccon E.…Neogi U. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microb. Infect. 2020;9(1):1748–1760. doi: 10.1080/22221751.2020.1799723. - DOI - PMC - PubMed
-
- Baildya N., Khan A.A., Ghosh N.N., Dutta T., Chattopadhyay A.P. Screening of potential drug from Azadirachta Indica (Neem) extracts for SARS-CoV-2: an insight from molecular docking and MD-simulation studies. J. Mol. Struct. 2021;1227:129390. doi: 10.1016/j.molstruc.2020.129390. - DOI - PMC - PubMed
-
- Bandyopadhyay U., Biswas K., Chatterjee R., Bandyopadhyay D., Chattopadhyay I., Ganguly C.K.…Banerjee R.K. Gastroprotective effect of Neem (Azadirachta indica) bark extract: possible involvement of H(+)-K(+)-ATPase inhibition and scavenging of hydroxyl radical. Life Sci. 2002;71(24):2845–2865. doi: 10.1016/s0024-3205(02)02143-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

