Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment
- PMID: 35219244
- PMCID: PMC8881716
- DOI: 10.1016/j.esmoop.2022.100404
Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment
Abstract
Background: Drug-induced interstitial lung disease (DIILD) is a form of interstitial lung disease resulting from exposure to drugs causing inflammation and possibly interstitial fibrosis. Antineoplastic drugs are the primary cause of DIILD, accounting for 23%-51% of cases, with bleomycin, everolimus, erlotinib, trastuzumab-deruxtecan and immune checkpoint inhibitors being the most common causative agents. DIILD can be difficult to identify and manage, and there are currently no specific guidelines on the diagnosis and treatment of DIILD caused by anticancer drugs.
Objective: To develop recommendations for the diagnosis and management of DIILD in cancer patients.
Methods: Based on the published literature and their clinical expertise, a multidisciplinary group of experts in Italy developed recommendations stratified by DIILD severity, based on the Common Terminology Criteria for Adverse Events.
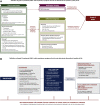
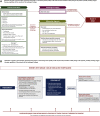
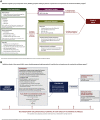
Results: The recommendations highlight the importance of multidisciplinary interaction in the diagnosis and management of DIILD. Important components of the diagnostic process are physical examination and careful patient history-taking, measurement of vital signs (particularly respiratory rate and arterial oxygen saturation), relevant laboratory tests, respiratory function testing with spirometry and diffusing capacity of the lung for carbon monoxide and computed tomography/imaging. Because the clinical and radiological signs of DIILD are often similar to those of pneumonias or interstitial lung diseases, differential diagnosis is important, including microbial and serological testing to exclude or confirm infectious causes. In most cases, management of DIILD requires the discontinuation of the antineoplastic agent and the administration of short-term steroids. Steroid tapering must be undertaken slowly to prevent reactivation of DIILD. Patients with severe and very severe (grade 3 and 4) DIILD will require hospitalisation and often need oxygen and non-invasive ventilation. Decisions about invasive ventilation should take into account the patient's cancer prognosis.
Conclusions: These recommendations provide a structured step-by-step diagnostic and therapeutic approach for each grade of suspected cancer-related DIILD.
Keywords: antineoplastic agents; diagnostic–therapeutic algorithm; differential diagnosis; interstitial lung disease; pneumonia.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure PC has received honoraria for consultation from Daiichi Sankyo. PAA has received grants and honoraria for consultation from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Idera Pharmaceuticals, Immunocore, Italfarmaco, iTeos Therapeutics, Lunaphore Technologies, Merck Serono, MSD, Nektar Therapeutics, Nouscom, Novartis, OncoSec, Pfizer, Pierre Fabre Pharma, 4SC, Regeneron Pharmaceuticals, Roche-Genentech, Sandoz, Sanofi, Seagen and Sun Pharma. RD has received grants and honoraria for consultation from AstraZeneca, Eli Lilly, Gilead Sciences, GlaxoSmithKline, Novartis and Pfizer. PT has received grants and honoraria for consultation from Daiichi Sankyo, Incyte, MedicAir and Vertex. AC has received honoraria for consultation from Angelini, Gilead Sciences and Janssen. MDL has received honoraria for consultation from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Exact Sciences, Gilead Sciences, MSD, Novartis, Pfizer, Pierre Fabre Pharma, Roche-Genentech and Seagen. AF has received grants and honoraria for consultation from Amgen, Bayer HealthCare Pharmaceuticals, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Incyte, Merck Serono, MSD, Pierre Fabre Pharma, Roche-Genentech and Servier. LR has received grants and honoraria for consultation from Biogen, Boehringer Ingelheim, Celgene Corporation, Cipla, CSL Behring, FibroGen, Nitto BioPharma, Pliant Therapeutics, Promedior, Respivant Sciences, Roche-Genentech, Sanofi Aventis and Zambon. SS has received grants and honoraria for consultation from Agenus, AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol Myers Squibb, CheckMab, Daiichi Sankyo, Merck Serono and Seagen. The remaining authors have declared no conflicts of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

