A selective inhibitor of the NLRP3 inflammasome as a potential therapeutic approach for neuroprotection in a transgenic mouse model of Huntington's disease
- PMID: 35219323
- PMCID: PMC8882273
- DOI: 10.1186/s12974-022-02419-9
A selective inhibitor of the NLRP3 inflammasome as a potential therapeutic approach for neuroprotection in a transgenic mouse model of Huntington's disease
Abstract
Background: Huntington's disease (HD) is a neurodegenerative disorder caused by the expansion of the CAG repeat in the huntingtin (HTT) gene. When the number of CAG repeats exceeds 36, the translated expanded polyglutamine-containing HTT protein (mutant HTT [mHTT]) interferes with the normal functions of many cellular proteins and subsequently jeopardizes important cellular machineries in major types of brain cells, including neurons, astrocytes, and microglia. The NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome, which comprises NLRP3, ASC, and caspase-1, is involved in the activation of IL-1β and IL-18 and has been implicated in various biological functions. Although the existence of the NLRP3 inflammasome in the brain has been documented, the roles of the NLRP3 inflammasome in HD remain largely uncharacterized. MCC950 is a highly selective and potent small-molecule inhibitor of NLRP3 that has been used for the treatment of several diseases such as Alzheimer's disease. However, whether MCC950 is also beneficial in HD remains unknown. Therefore, we hypothesized that MCC950 exerts beneficial effects in a transgenic mouse model of HD.
Methods: To evaluate the effects of MCC950 in HD, we used the R6/2 (B6CBA-Tg[HDexon1]62Gpb/1J) transgenic mouse model of HD, which expresses exon 1 of the human HTT gene carrying 120 ± 5 CAG repeats. Male transgenic R6/2 mice were treated daily with MCC950 (10 mg/kg of body weight; oral administration) or water for 5 weeks from the age of 7 weeks. We examined neuronal density, neuroinflammation, and mHTT aggregation in the striatum of R6/2 mice vs. their wild-type littermates. We also evaluated the motor function, body weight, and lifespan of R6/2 mice.
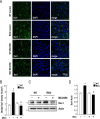
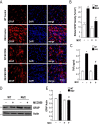
Results: Systematic administration of MCC950 to R6/2 mice suppressed the NLRP3 inflammasome, decreased IL-1β and reactive oxygen species production, and reduced neuronal toxicity, as assessed based on increased neuronal density and upregulation of the NeuN and PSD-95 proteins. Most importantly, oral administration of MCC950 increased neuronal survival, reduced neuroinflammation, extended lifespan, and improved motor dysfunction in R6/2 mice.
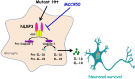
Conclusions: Collectively, our findings indicate that MCC950 exerts beneficial effects in a transgenic mouse model of HD and has therapeutic potential for treatment of this devastating neurodegenerative disease.
Keywords: Huntington’s disease (HD); Interleukin‐1β (IL‐1β); Mutated huntingtin (mHTT); Nucleotide oligomerization domain-like receptor protein 3 inflammasome (NLRP3 inflammasome).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Protective Effects of Antcin H Isolated from Antrodia cinnamomea Against Neuroinflammation in Huntington's Disease via NLRP3 Inflammasome Inhibition.J Neuroimmune Pharmacol. 2024 Dec 2;20(1):1. doi: 10.1007/s11481-024-10161-7. J Neuroimmune Pharmacol. 2024. PMID: 39621196
-
Parthenolide ameliorates 3-nitropropionic acid-induced Huntington's disease-like aberrations via modulating NLRP3 inflammasome, reducing microglial activation and inducing astrocyte shifting.Mol Med. 2024 Sep 26;30(1):158. doi: 10.1186/s10020-024-00917-5. Mol Med. 2024. PMID: 39327568 Free PMC article.
-
Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury.Neurobiol Dis. 2018 Sep;117:15-27. doi: 10.1016/j.nbd.2018.05.016. Epub 2018 May 30. Neurobiol Dis. 2018. PMID: 29859317
-
Huntington's disease mouse models: unraveling the pathology caused by CAG repeat expansion.Fac Rev. 2021 Oct 21;10:77. doi: 10.12703/r/10-77. eCollection 2021. Fac Rev. 2021. PMID: 34746930 Free PMC article. Review.
-
Inflammasome activation and assembly in Huntington's disease.Mol Immunol. 2022 Nov;151:134-142. doi: 10.1016/j.molimm.2022.09.002. Epub 2022 Sep 18. Mol Immunol. 2022. PMID: 36126501 Review.
Cited by
-
Mitochondria and sensory processing in inflammatory and neuropathic pain.Front Pain Res (Lausanne). 2022 Oct 17;3:1013577. doi: 10.3389/fpain.2022.1013577. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 36324872 Free PMC article. Review.
-
Interactions Between the Ubiquitin-Proteasome System, Nrf2, and the Cannabinoidome as Protective Strategies to Combat Neurodegeneration: Review on Experimental Evidence.Neurotox Res. 2024 Feb 23;42(2):18. doi: 10.1007/s12640-024-00694-3. Neurotox Res. 2024. PMID: 38393521 Free PMC article. Review.
-
Advances in Huntington's Disease Biomarkers: A 10-Year Bibliometric Analysis and a Comprehensive Review.Biology (Basel). 2025 Jan 26;14(2):129. doi: 10.3390/biology14020129. Biology (Basel). 2025. PMID: 40001897 Free PMC article. Review.
-
Protective Effects of Antcin H Isolated from Antrodia cinnamomea Against Neuroinflammation in Huntington's Disease via NLRP3 Inflammasome Inhibition.J Neuroimmune Pharmacol. 2024 Dec 2;20(1):1. doi: 10.1007/s11481-024-10161-7. J Neuroimmune Pharmacol. 2024. PMID: 39621196
-
Beyond Amyloid and Tau: The Critical Role of Microglia in Alzheimer's Disease Therapeutics.Biomedicines. 2025 Jan 23;13(2):279. doi: 10.3390/biomedicines13020279. Biomedicines. 2025. PMID: 40002692 Free PMC article. Review.
References
-
- Martin JB, Gusella JF. Huntington's disease. Pathogenesis and management. N Engl J Med. 1986;315:1267–1276. - PubMed
-
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559–577. - PubMed
-
- T. H. s. D. C. R. Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

