Need for aligning the definition and reporting of cytokine release syndrome (CRS) in immuno-oncology clinical trials
- PMID: 35219582
- PMCID: PMC9721456
- DOI: 10.1016/j.jcyt.2022.01.004
Need for aligning the definition and reporting of cytokine release syndrome (CRS) in immuno-oncology clinical trials
Abstract
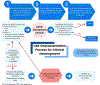
As cancer immunotherapies continue to expand across all areas of oncology, it is imperative to establish a standardized approach for defining and capturing clinically important toxicities, such as cytokine release syndrome (CRS). In this paper, we provide considerations for categorizing the variety of adverse events that may accompany CRS and for recognizing that presentations of CRS may differ among various immunotherapies (e.g., monoclonal antibodies, CAR T cell therapies and T cell engagers, which can include bispecific antibodies and other constructs). The goals of this paper are to ensure accurate and consistent identification of CRS in patients receiving immunotherapies in clinical studies to aid in reporting; enable more precise evaluation of the therapeutic risk-benefit profile and cross-study analyses; support evidence-based monitoring and management of important toxicities related to cancer immunotherapies; and improve patient care and outcomes. These efforts will become more important as the number and variety of molecular targets for immunotherapies broaden and as therapies with novel mechanisms continue to be developed.
Keywords: Cytokine Release Syndrome (CRS); Drug development; Immunotherapies; T cell receptors.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of Interest
B.M. is an employee of Genentech and owner of Roche stock. A.S.Y. is an employee of Xencor and owner of Xencor stock. C.D.B. is an employee of Amgen and owner of Amgen stock. M.M.H. has received grant funding for research from Allovir, Amgen, Astellas, Gamida Cell Ltd., Genentech Inc., Magenta Therapeutics, Medac GmbH, OncImmune, and Vor Biopharma. C.M. is an employee of BMS and owner of BMS and Amgen stocks. P.K.M. is an employee of Amgen and owner of Amgen stock. E.O. is an employee of Regeneron. R.R. is an employee of Bristol Myers Squibb. F.V. is an employee of Bristol Myers Squibb. T. Y. is an employee of Amgen and owner of Amgen stock. All other authors have no disclosures to report.
Figures

References
-
- Xin Yu J, Hubbard-Lucey VM, Tang J. Immuno-oncology drug development goes global. Nature reviews. Drug discovery 2019;18:899–900. - PubMed
-
- Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy 2019;11:851–7. - PubMed
-
- Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39:3978–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

