Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1
- PMID: 35219746
- PMCID: PMC8980959
- DOI: 10.1016/j.jlr.2022.100187
Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1
Abstract
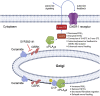
The sphingolipid, ceramide-1-phosphate (C1P), has been shown to promote the inflammatory phase and inhibit the proliferation and remodeling stages of wound repair via direct interaction with group IVA cytosolic phospholipase A2, a regulator of eicosanoid biosynthesis that fine-tunes the behaviors of various cell types during wound healing. However, the anabolic enzyme responsible for the production of C1P that suppresses wound healing as well as bioactive eicosanoids and target receptors that drive enhanced wound remodeling have not been characterized. Herein, we determined that decreasing C1P activity via inhibitors or genetic ablation of the anabolic enzyme ceramide kinase (CERK) significantly enhanced wound healing phenotypes. Importantly, postwounding inhibition of CERK enhanced the closure rate of acute wounds, improved the quality of healing, and increased fibroblast migration via a "class switch" in the eicosanoid profile. This switch reduced pro-inflammatory prostaglandins (e.g., prostaglandin E2) and increased levels of 5-hydroxyeicosatetraenoic acid and the downstream metabolite 5-oxo-eicosatetraenoic acid (5-oxo-ETE). Moreover, dermal fibroblasts from mice with genetically ablated CERK showed enhanced wound healing markers, while blockage of the murine 5-oxo-ETE receptor (oxoeicosanoid receptor 1) inhibited the enhanced migration phenotype of these cell models. Together, these studies reinforce the vital roles eicosanoids play in the wound healing process and demonstrate a novel role for CERK-derived C1P as a negative regulator of 5-oxo-ETE biosynthesis and the activation of oxoeicosanoid receptor 1 in wound healing. These findings provide foundational preclinical results for the use of CERK inhibitors to shift the balance from inflammation to resolution and increase the wound healing rate.
Keywords: 5-HETE; 5-oxo-ETE; arachidonic acid; ceramide kinase; ceramide-1-phosphate; eicosanoids; group IVA phospholipases A2; inflammation; lipidomics.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


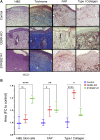
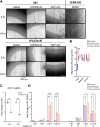
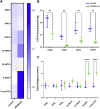
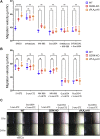
Similar articles
-
Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts.J Lipid Res. 2014 Jul;55(7):1298-309. doi: 10.1194/jlr.M048207. Epub 2014 May 13. J Lipid Res. 2014. PMID: 24823941 Free PMC article.
-
The interaction of ceramide 1-phosphate with group IVA cytosolic phospholipase A2 coordinates acute wound healing and repair.Sci Signal. 2019 Dec 3;12(610):eaav5918. doi: 10.1126/scisignal.aav5918. Sci Signal. 2019. PMID: 31796632 Free PMC article.
-
Characterization of eicosanoid synthesis in a genetic ablation model of ceramide kinase.J Lipid Res. 2013 Jul;54(7):1834-47. doi: 10.1194/jlr.M035683. Epub 2013 Apr 10. J Lipid Res. 2013. PMID: 23576683 Free PMC article.
-
Novel signaling aspects of ceramide 1-phosphate.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Apr;1865(4):158630. doi: 10.1016/j.bbalip.2020.158630. Epub 2020 Jan 17. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31958571 Review.
-
Ceramide kinase and the ceramide-1-phosphate/cPLA2alpha interaction as a therapeutic target.Curr Drug Targets. 2008 Aug;9(8):674-82. doi: 10.2174/138945008785132349. Curr Drug Targets. 2008. PMID: 18691014 Review.
Cited by
-
Unraveling the molecular dynamics of wound healing: integrating spatially resolved lipidomics and temporally resolved proteomics.Anal Bioanal Chem. 2025 Jun;417(15):3299-3314. doi: 10.1007/s00216-025-05865-5. Epub 2025 Apr 24. Anal Bioanal Chem. 2025. PMID: 40272507 Free PMC article.
-
RNA splicing variants of the novel long non-coding RNA, CyKILR, possess divergent biological functions in non-small cell lung cancer.Mol Ther Nucleic Acids. 2024 Dec 5;36(1):102412. doi: 10.1016/j.omtn.2024.102412. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2024. PMID: 39807365 Free PMC article.
-
Small extracellular vesicles (sEVs) in pancreatic cancer progression and diagnosis.J Control Release. 2025 Apr 10;380:269-282. doi: 10.1016/j.jconrel.2025.01.072. Epub 2025 Feb 6. J Control Release. 2025. PMID: 39889882 Review.
-
Differential lipid signaling from CD4+ and CD8+ T cells contributes to type 1 diabetes development.Front Immunol. 2024 Sep 18;15:1444639. doi: 10.3389/fimmu.2024.1444639. eCollection 2024. Front Immunol. 2024. PMID: 39359722 Free PMC article.
-
Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process.Int J Mol Sci. 2024 Mar 28;25(7):3790. doi: 10.3390/ijms25073790. Int J Mol Sci. 2024. PMID: 38612601 Free PMC article. Review.
References
-
- Clark R.A.F. In: The Molecular and Cellular Biology of Wound Repair. Clark R.A.F., editor. Springer; Boston, MA: 1988. Wound repair; pp. 3–50. Chapter 1.
-
- Versteeg H.H., Heemskerk J.W.M., Levi M., Reitsma P.H. New fundamentals in hemostasis. Physiol. Rev. 2013;93:327–358. - PubMed
-
- Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 2004;9:283–289. - PubMed
-
- Broughton G., Janis J.E., Attinger C.E. Wound healing: an overview. Plast. Reconstr. Surg. 2006;117:1e-S–32e-S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

