The Biphasic Effect of Retinoic Acid Signaling Pathway on the Biased Differentiation of Atrial-like and Sinoatrial Node-like Cells from hiPSC
- PMID: 35220280
- PMCID: PMC9396015
- DOI: 10.15283/ijsc21148
The Biphasic Effect of Retinoic Acid Signaling Pathway on the Biased Differentiation of Atrial-like and Sinoatrial Node-like Cells from hiPSC
Abstract
Background and objectives: Although human-induced pluripotent stem cells (hiPSC) can be efficiently differentiated into cardiomyocytes (CMs), the heterogeneity of the hiPSC-CMs hampers their applications in research and regenerative medicine. Retinoic acid (RA)-mediated signaling pathway has been proved indispensable in cardiac development and differentiation of hiPSC toward atrial CMs. This study was aimed to test whether RA signaling pathway can be manipulated to direct the differentiation into sinoatrial node (SAN) CMs.
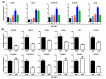
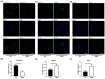
Methods and results: Using the well-characterized GiWi protocol that cardiomyocytes are generated from hiPSC via temporal modulation of Wnt signaling pathway by small molecules, RA signaling pathway was manipulated during the differentiation of hiPSC-CMs on day 5 post-differentiation, a crucial time point equivalent to the transition from cardiac mesoderm to cardiac progenitor cells in cardiac development. The resultant CMs were characterized at mRNA, protein and electrophysiology levels by a combination of qPCR, immunofluorescence, flow cytometry, and whole-cell patch clamp. The results showed that activation of the RA signaling pathway biased the differentiation of atrial CMs, whereas inhibition of the signaling pathway biased the differentiation of sinoatrial node-like cells (SANLCs).
Conclusions: Our study not only provides a novel and simple strategy to enrich SANLCs but also improves our understanding of the importance of RA signaling in the differentiation of hiPSC-CMs.
Keywords: Atrial-like cells; Biased differentiation; Human-induced pluripotent stem cell (hiPSC); Retinoic acid (RA) signaling; Sinoatrial node-like cells (SANLCs).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Enrichment differentiation of human induced pluripotent stem cells into sinoatrial node-like cells by combined modulation of BMP, FGF, and RA signaling pathways.Stem Cell Res Ther. 2020 Jul 16;11(1):284. doi: 10.1186/s13287-020-01794-5. Stem Cell Res Ther. 2020. PMID: 32678003 Free PMC article.
-
Chemically defined and small molecules-based generation of sinoatrial node-like cells.Stem Cell Res Ther. 2022 Apr 11;13(1):158. doi: 10.1186/s13287-022-02834-y. Stem Cell Res Ther. 2022. PMID: 35410454 Free PMC article.
-
Patch-Clamp Recording from Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Improving Action Potential Characteristics through Dynamic Clamp.Int J Mol Sci. 2017 Aug 30;18(9):1873. doi: 10.3390/ijms18091873. Int J Mol Sci. 2017. PMID: 28867785 Free PMC article.
-
Production of functional cardiomyocytes and cardiac tissue from human induced pluripotent stem cells for regenerative therapy.J Mol Cell Cardiol. 2022 Mar;164:83-91. doi: 10.1016/j.yjmcc.2021.11.008. Epub 2021 Nov 22. J Mol Cell Cardiol. 2022. PMID: 34822838 Review.
-
State-of-the-Art Differentiation Protocols for Patient-Derived Cardiac Pacemaker Cells.Int J Mol Sci. 2024 Mar 16;25(6):3387. doi: 10.3390/ijms25063387. Int J Mol Sci. 2024. PMID: 38542361 Free PMC article. Review.
Cited by
-
Differentiation of Sinoatrial-like Cardiomyocytes as a Biological Pacemaker Model.Int J Mol Sci. 2024 Aug 23;25(17):9155. doi: 10.3390/ijms25179155. Int J Mol Sci. 2024. PMID: 39273104 Free PMC article. Review.
-
The Current State of Realistic Heart Models for Disease Modelling and Cardiotoxicity.Int J Mol Sci. 2024 Aug 24;25(17):9186. doi: 10.3390/ijms25179186. Int J Mol Sci. 2024. PMID: 39273136 Free PMC article. Review.
-
Generation of cardiomyocytes from human-induced pluripotent stem cells resembling atrial cells with ability to respond to adrenoceptor agonists.Philos Trans R Soc Lond B Biol Sci. 2023 Jun 19;378(1879):20220312. doi: 10.1098/rstb.2022.0312. Epub 2023 May 1. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37122218 Free PMC article.
References
LinkOut - more resources
Full Text Sources

