A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- PMID: 35220295
- PMCID: PMC8889334
- DOI: 10.15283/ijsc21195
A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
Abstract
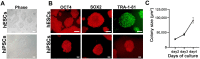
Background and objectives: In recent years, brain organoid technologies have been the most innovative advance in neural differentiation research. In line with this, we optimized a method to establish cerebral organoids from feeder-free cultured human pluripotent stem cells. In this study, we focused on the consistent and robust production of cerebral organoids comprising neural progenitor cells and neurons. We propose an optimal protocol for cerebral organoid generation that is applicable to both human embryonic stem cells and human induced pluripotent stem cells.
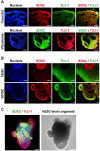
Methods and results: We investigated formation of neuroepithelium, neural tube, and neural folding by observing the morphology of embryoid bodies at each stage during the cerebral organoid differentiation process. Furthermore, we characterized the cerebral organoids via immunocytochemical staining of sectioned organoid samples, which were prepared using a Cryostat and Vibratome. Finally, we established a routine method to generate early cerebral organoids comprising a cortical layer and a neural progenitor zone.
Conclusions: We developed an optimized methodology for the generation of cerebral organoids using hESCs and hiPSCs. Using this protocol, consistent and efficient cerebral organoids could be obtained from hiPSCs as well as hESCs. Further, the morphology of brain organoids could be analyzed through 2D monitoring via immunostaining and tissue sectioning, or through 3D monitoring by whole tissue staining after clarification.
Keywords: Brain organoid; Differentiation; Human pluripotent stem cells; Organoid; Pluripotency.
Conflict of interest statement
The authors have no conflicting financial interest.
Figures


References
LinkOut - more resources
Full Text Sources

