Efficacy and Safety of a New Sustained-release Pregabalin Formulation Compared With Immediate-release Pregabalin in Patients With Peripheral Neuropathic Pain: A Randomized Noninferiority Phase 3 Trial
- PMID: 35220330
- PMCID: PMC8994039
- DOI: 10.1097/AJP.0000000000001028
Efficacy and Safety of a New Sustained-release Pregabalin Formulation Compared With Immediate-release Pregabalin in Patients With Peripheral Neuropathic Pain: A Randomized Noninferiority Phase 3 Trial
Abstract
Objective: This study investigated whether a new sustained-release (SR) pregabalin formulation is noninferior to immediate-release (IR) pregabalin in alleviating peripheral neuropathic pain in Korean patients.
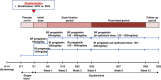
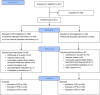
Materials and methods: This was a randomized, double-blind, active-controlled phase 3 study of patients with diabetic peripheral neuropathy or postherpetic neuralgia from 41 sites in South Korea in 2017-2018. Eligible patients were randomized (1:1) to receive once-daily SR pregabalin or twice-daily IR pregabalin (150 to 600 mg/d) in a double-dummy manner for 12 weeks according to a stratified permuted block randomization scheme. The primary endpoint was the Daily Pain Rating Scale score at the end of treatment, averaged from the last 7 available scores.
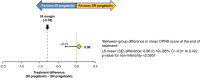
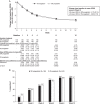
Results: A total of 319 of 371 (86.0%) randomized patients completed the 12-week treatment (SR pregabalin: n=154; IR pregabalin: n=165; per-protocol set: n=296). The least square mean difference between both groups for the primary endpoint was 0.06 (SE 0.19); (95% confidence interval -0.31 to 0.42), with the lower limit of the confidence interval above the pre-specified margin (-0.78; Pnoninferiority<0.0001). Drug-related treatment-emergent adverse events (TEAEs) were comparable between both groups. The incidence of drug-related TEAEs leading to treatment discontinuation was low (SR pregabalin: 2.7%; IR pregabalin: 1.1%). No serious drug-related TEAEs or deaths occurred.
Discussion: The results demonstrate that the new once-daily SR pregabalin formulation is noninferior to twice-daily IR pregabalin in reducing peripheral neuropathic pain and is well tolerated in Korean patients with diabetic peripheral neuropathy or postherpetic neuralgia after 12 weeks of treatment.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Supported by Yuhan Corporation (Seoul, Republic of Korea), which was involved in all aspects of study design, data collection, data analysis, data interpretation, assisted in writing the report, and approved the final version of the manuscript for publication in conjunction with the authors. S.K. and T.A. are employees of Yuhan Corporation, Seoul, Republic of Korea. The remaining authors declare no conflict of interest.
Figures
References
-
- Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012;16:191–198. - PubMed
-
- International Association for the Study of Pain (IASP). IASP terminology. Available at: www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698#Peripheralneuro.... Accessed September 6, 2020.
-
- Dworkin RH, Malone DC, Panarites CJ, et al. . Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on health care costs. J Pain. 2010;11:360–368. - PubMed
-
- Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–674. - PubMed
-
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

