Targeting the Erk1/2 and autophagy signaling easily improved the neurobalst differentiation and cognitive function after young transient forebrain ischemia compared to old gerbils
- PMID: 35220404
- PMCID: PMC8882190
- DOI: 10.1038/s41420-022-00888-8
Targeting the Erk1/2 and autophagy signaling easily improved the neurobalst differentiation and cognitive function after young transient forebrain ischemia compared to old gerbils
Abstract
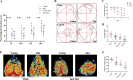
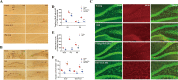
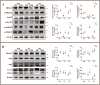
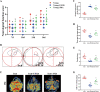
The hippocampal neurogenesis occurs constitutively throughout adulthood in mammalian species, but declines with age. In this study, we overtly found that the neuroblast proliferation and differentiation in the subgranular zone and the maturation into fully functional and integrated neurons in the granule-cell layer in young gerbils following cerebral ischemia/reperfusion was much more than those in old gerbils. The neurological function and cognitive and memory-function rehabilitation in the young gerbils improved faster than those in the old one. These results demonstrated that, during long term after cerebral ischemia/reperfusion, the ability of neurogenesis and recovery of nerve function in young animals were significantly higher than that in the old animals. We found that, after 14- and 28-day cerebral ischemia/reperfusion, the phosphorylation of MEK1/2, ERK1/2, p90RSK, and MSK1/2 protein levels in the hippocampus of young gerbils was significantly much higher than that of old gerbils. The levels of autophagy-related proteins, including Beclin-1, Atg3, Atg5, and LC3 in the hippocampus were effectively maintained and elevated at 28 days after cerebral ischemia/reperfusion in the young gerbils compared with those in the old gerbils. These results indicated that an increase or maintenance of the phosphorylation of ERK1/2 signal pathway and autophagy-related proteins was closely associated with the neuroblast proliferation and differentiation and the process of maturation into neurons. Further, we proved that neuroblast proliferation and differentiation in the dentate gyrus and cognitive function were significantly reversed in young cerebral ischemic gerbils by administering the ERK inhibitor (U0126) and autophagy inhibitor (3MA). In brief, following experimental young ischemic stroke, the long-term promotion of the neurogenesis in the young gerbil's hippocampal dentate gyrus by upregulating the phosphorylation of ERK signaling pathway and maintaining autophagy-related protein levels, it overtly improved the neurological function and cognitive and memory function.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Differences in TNF‑α and TNF‑R1 expression in damaged neurons and activated astrocytes of the hippocampal CA1 region between young and adult gerbils following transient forebrain ischemia.Mol Med Rep. 2021 Sep;24(3):625. doi: 10.3892/mmr.2021.12264. Epub 2021 Jul 2. Mol Med Rep. 2021. PMID: 34212986 Free PMC article.
-
Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils.J Neurosci. 1998 Oct 1;18(19):7768-78. doi: 10.1523/JNEUROSCI.18-19-07768.1998. J Neurosci. 1998. PMID: 9742147 Free PMC article.
-
Inhibitor of vascular endothelial growth factor receptor tyrosine kinase attenuates cellular proliferation and differentiation to mature neurons in the hippocampal dentate gyrus after transient forebrain ischemia in the adult rat.Neuroscience. 2006 Sep 1;141(3):1209-16. doi: 10.1016/j.neuroscience.2006.04.064. Epub 2006 Jun 6. Neuroscience. 2006. PMID: 16753262
-
Differential expression of HSC73 and HSP72 mRNA and proteins between young and adult gerbils after transient cerebral ischemia: relation to neuronal vulnerability.J Cereb Blood Flow Metab. 2000 Jul;20(7):1056-65. doi: 10.1097/00004647-200007000-00005. J Cereb Blood Flow Metab. 2000. PMID: 10908039
-
Recombinant human thioredoxin-1 promotes neurogenesis and facilitates cognitive recovery following cerebral ischemia in mice.Neuropharmacology. 2014 Feb;77:453-64. doi: 10.1016/j.neuropharm.2013.10.027. Epub 2013 Nov 7. Neuropharmacology. 2014. PMID: 24212059
Cited by
-
Changes of Sonic Hedgehog mediated FAK/ERK pathway proteins in amyotrophic lateral sclerosis model mice.Psychopharmacology (Berl). 2025 Jul 4. doi: 10.1007/s00213-025-06846-3. Online ahead of print. Psychopharmacology (Berl). 2025. PMID: 40613930 No abstract available.
-
Fe3O4 Nanozymes Improve Neuroblast Differentiation and Blood-Brain Barrier Integrity of the Hippocampal Dentate Gyrus in D-Galactose-Induced Aged Mice.Int J Mol Sci. 2022 Jun 9;23(12):6463. doi: 10.3390/ijms23126463. Int J Mol Sci. 2022. PMID: 35742908 Free PMC article.
-
Brain washing and neural health: role of age, sleep, and the cerebrospinal fluid melatonin rhythm.Cell Mol Life Sci. 2023 Mar 14;80(4):88. doi: 10.1007/s00018-023-04736-5. Cell Mol Life Sci. 2023. PMID: 36917314 Free PMC article. Review.
-
Adeno-associated Virus-mediated Ezh2 Knockdown Reduced the Increment of Newborn Neurons Induced by Forebrain Ischemia in Gerbil Dentate Gyrus.Mol Neurobiol. 2024 Nov;61(11):9623-9632. doi: 10.1007/s12035-024-04200-w. Epub 2024 Apr 27. Mol Neurobiol. 2024. PMID: 38676810 Free PMC article.
References
-
- Meng H, Jin W, Yu L, Xu S, Wan H, He Y. Protective effects of polysaccharides on cerebral ischemia: A mini-review of the mechanisms. Int J Biol Macromol. 2021;169:463–72. - PubMed
-
- Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. 2017;8:3–13. - PubMed
-
- Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019;92:e2444–e54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

