Targeted siRNA nanocarrier: a platform technology for cancer treatment
- PMID: 35220407
- PMCID: PMC8993695
- DOI: 10.1038/s41388-022-02241-w
Targeted siRNA nanocarrier: a platform technology for cancer treatment
Abstract
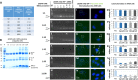
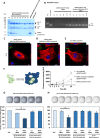
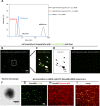
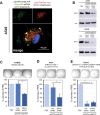
The small arginine-rich protein protamine condenses complete genomic DNA into the sperm head. Here, we applied its high RNA binding capacity for spontaneous electrostatic assembly of therapeutic nanoparticles decorated with tumour-cell-specific antibodies for efficiently targeting siRNA. Fluorescence microscopy and DLS measurements of these nanocarriers revealed the formation of a vesicular architecture that requires presence of antibody-protamine, defined excess of free SMCC-protamine, and anionic siRNA to form. Only these complex nanoparticles were efficient in the treatment of non-small-cell lung cancer (NSCLC) xenograft models, when the oncogene KRAS was targeted via EGFR-mediated delivery. To show general applicability, we used the modular platform for IGF1R-positive Ewing sarcomas. Anti-IGR1R-antibodies were integrated into an antibody-protamine nanoparticle with an siRNA specifically against the oncogenic translocation product EWS/FLI1. Using these nanoparticles, EWS/FLI1 knockdown blocked in vitro and in vivo growth of Ewing sarcoma cells. We conclude that these antibody-protamine-siRNA nanocarriers provide a novel platform technology to specifically target different cell types and yet undruggable targets in cancer therapy by RNAi.
© 2022. The Author(s).
Conflict of interest statement
NB, LW, GL, WEB, and SB have filed two patent applications on electrostatic nanocarrier technology. All other authors disclose no conflict of interest.
Figures



References
-
- Carr JA, Silverman N. The heparin-protamine interaction. A review. J Cardiovasc Surg. 1999;40:659–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

