Insular cortex corticotropin-releasing factor integrates stress signaling with social affective behavior
- PMID: 35220413
- PMCID: PMC9018766
- DOI: 10.1038/s41386-022-01292-7
Insular cortex corticotropin-releasing factor integrates stress signaling with social affective behavior
Abstract
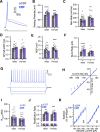
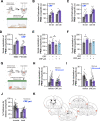
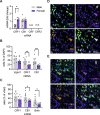
Impairments in identifying and responding to the emotions of others manifest in a variety of psychopathologies. Therefore, elaborating the neurobiological mechanisms that underpin social responses to social emotions, or social affective behavior, is a translationally important goal. The insular cortex is consistently implicated in stress-related social and anxiety disorders, which are associated with diminished ability to make and use inferences about the emotions of others to guide behavior. We investigated how corticotropin-releasing factor (CRF), a neuromodulator evoked upon exposure to stressed conspecifics, influenced the insula. We hypothesized that social affective behavior requires CRF signaling in the insular cortex in order to detect stress in social interactions. In acute slices from male and female rats, CRF depolarized insular pyramidal neurons. In males, but not females, CRF suppressed presynaptic GABAergic inhibition leading to greater excitatory synaptic efficacy in a CRF receptor 1 (CRF1)- and cannabinoid receptor 1 (CB1)-dependent fashion. In males only, insular CRF increased social investigation, and CRF1 and CB1 antagonists interfered with social interactions with stressed conspecifics. To investigate the molecular and cellular basis for the effect of CRF we examined insular CRF1 and CB1 mRNAs and found greater total insula CRF1 mRNA in females but greater CRF1 and CB1 mRNA colocalization in male insular cortex glutamatergic neurons that suggest complex, sex-specific organization of CRF and endocannabinoid systems. Together these results reveal a new mechanism by which stress and affect contribute to social affective behavior.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Maternal immune activation alters social affective behavior and sensitivity to corticotropin releasing factor in male but not female rats.Horm Behav. 2023 Mar;149:105313. doi: 10.1016/j.yhbeh.2023.105313. Epub 2023 Jan 25. Horm Behav. 2023. PMID: 36706685 Free PMC article.
-
Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons.Neuropharmacology. 2020 Dec 1;180:108296. doi: 10.1016/j.neuropharm.2020.108296. Epub 2020 Sep 17. Neuropharmacology. 2020. PMID: 32950560 Free PMC article.
-
Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype.Biol Psychiatry. 2017 Oct 1;82(7):500-510. doi: 10.1016/j.biopsych.2017.01.005. Epub 2017 Jan 13. Biol Psychiatry. 2017. PMID: 28209423 Free PMC article.
-
Corticotrophin-releasing factor 1 activation in the central amygdale and visceral hyperalgesia.Neurogastroenterol Motil. 2015 Jan;27(1):1-6. doi: 10.1111/nmo.12495. Neurogastroenterol Motil. 2015. PMID: 25557223 Free PMC article. Review.
-
Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents.Gend Med. 2005 Sep;2(3):146-54. doi: 10.1016/s1550-8579(05)80043-9. Gend Med. 2005. PMID: 16290887 Review.
Cited by
-
Olfactory bulb astrocytes link social transmission of stress to cognitive adaptation in male mice.Nat Commun. 2024 Aug 18;15(1):7103. doi: 10.1038/s41467-024-51416-4. Nat Commun. 2024. PMID: 39155299 Free PMC article.
-
Social affective behaviors among female rats involve the basolateral amygdala and insular cortex.PLoS One. 2023 Oct 5;18(10):e0281794. doi: 10.1371/journal.pone.0281794. eCollection 2023. PLoS One. 2023. PMID: 37797037 Free PMC article.
-
Serotonin modulates social responses to stressed conspecifics via insular 5-HT2C receptors in rat.Neuropharmacology. 2023 Sep 15;236:109598. doi: 10.1016/j.neuropharm.2023.109598. Epub 2023 May 23. Neuropharmacology. 2023. PMID: 37230216 Free PMC article.
-
Maternal immune activation alters social affective behavior and sensitivity to corticotropin releasing factor in male but not female rats.Horm Behav. 2023 Mar;149:105313. doi: 10.1016/j.yhbeh.2023.105313. Epub 2023 Jan 25. Horm Behav. 2023. PMID: 36706685 Free PMC article.
-
Social affective behaviors among female rats involve the basolateral amygdala and insular cortex.bioRxiv [Preprint]. 2023 Feb 3:2023.02.02.526780. doi: 10.1101/2023.02.02.526780. bioRxiv. 2023. Update in: PLoS One. 2023 Oct 5;18(10):e0281794. doi: 10.1371/journal.pone.0281794. PMID: 36778382 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

