Sex-specific effects of bisphenol A on the signaling pathway of ESRRG in the human placenta†
- PMID: 35220427
- PMCID: PMC9198953
- DOI: 10.1093/biolre/ioac044
Sex-specific effects of bisphenol A on the signaling pathway of ESRRG in the human placenta†
Abstract
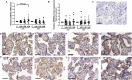
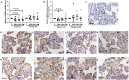
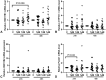
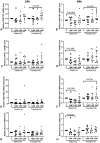
Bisphenol A (BPA) exposure during pregnancy is associated with low fetal weight, particularly in male fetuses. The expression of estrogen-related receptor gamma (ESRRG), a receptor for BPA in the human placenta, is reduced in fetal growth restriction. This study sought to explore whether ESRRG signaling mediates BPA-induced placental dysfunction and determine whether changes in the ESRRG signaling pathway are sex-specific. Placental villous explants from 18 normal term pregnancies were cultured with a range of BPA concentrations (1 nM-1 μM). Baseline BPA concentrations in the placental tissue used for explant culture ranged from 0.04 to 5.1 nM (average 2.3 ±1.9 nM; n = 6). Expression of ESRRG signaling pathway constituents and cell turnover were quantified. BPA (1 μM) increased ESRRG mRNA expression after 24 h in both sexes. ESRRG mRNA and protein expression was increased in female placentas treated with 1 μM BPA for 24 h but was decreased in male placentas treated with 1 nM or 1 μM for 48 h. Levels of 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1) and placenta specific-1 (PLAC1), genes downstream of ESRRG, were also affected. HSD17B1 mRNA expression was increased in female placentas by 1 μM BPA; however, 1 nM BPA reduced HSD17B1 and PLAC1 expression in male placentas at 48 h. BPA treatment did not affect rates of proliferation, apoptosis, or syncytiotrophoblast differentiation in cultured villous explants. This study has demonstrated that BPA affects the ESRRG signaling pathway in a sex-specific manner in human placentas and a possible biological mechanism to explain the differential effects of BPA exposure on male and female fetuses observed in epidemiological studies.
Keywords: bisphenol A; estrogen-related receptor gamma; human placenta; placental dysfunction; sex specific manner.
© The Author(s) 2022. Published by Oxford University Press behalf of Society for the Study of Reproduction.
Figures



Similar articles
-
Placental expression of estrogen-related receptor gamma is reduced in fetal growth restriction pregnancies and is mediated by hypoxia†.Biol Reprod. 2022 Sep 12;107(3):846-857. doi: 10.1093/biolre/ioac108. Biol Reprod. 2022. PMID: 35594451 Free PMC article.
-
Gestational bisphenol S impairs placental endocrine function and the fusogenic trophoblast signaling pathway.Arch Toxicol. 2018 May;92(5):1861-1876. doi: 10.1007/s00204-018-2191-2. Epub 2018 Mar 17. Arch Toxicol. 2018. PMID: 29550860 Free PMC article.
-
The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures.Mol Cell Endocrinol. 2016 Jul 5;429:41-9. doi: 10.1016/j.mce.2016.03.034. Epub 2016 Mar 30. Mol Cell Endocrinol. 2016. PMID: 27036933
-
The potential role of the E SRRG pathway in placental dysfunction.Reproduction. 2021 Mar;161(3):R45-R60. doi: 10.1530/REP-20-0272. Reproduction. 2021. PMID: 33361468 Review.
-
Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives.Toxicol Sci. 2018 Dec 1;166(2):250-268. doi: 10.1093/toxsci/kfy224. Toxicol Sci. 2018. PMID: 30203063 Free PMC article. Review.
Cited by
-
Short-Half-Life Chemicals: Maternal Exposure and Offspring Health Consequences-The Case of Synthetic Phenols, Parabens, and Phthalates.Toxics. 2024 Sep 29;12(10):710. doi: 10.3390/toxics12100710. Toxics. 2024. PMID: 39453131 Free PMC article. Review.
-
Effects of Bisphenol A on the Risk of Developing Obesity.Nutrients. 2024 Oct 31;16(21):3740. doi: 10.3390/nu16213740. Nutrients. 2024. PMID: 39519574 Free PMC article. Review.
-
Mechanisms of bisphenol A and its analogs as endocrine disruptors via nuclear receptors and related signaling pathways.Arch Toxicol. 2025 Jun;99(6):2397-2417. doi: 10.1007/s00204-025-04025-z. Epub 2025 Mar 21. Arch Toxicol. 2025. PMID: 40116906 Free PMC article. Review.
-
Distinct epigenetic modulation of differentially expressed genes in the adult mouse brain following prenatal exposure to low-dose bisphenol A.Cell Biol Toxicol. 2024 May 22;40(1):37. doi: 10.1007/s10565-024-09875-4. Cell Biol Toxicol. 2024. PMID: 38777957 Free PMC article.
-
Placental single cell transcriptomics: Opportunities for endocrine disrupting chemical toxicology.Mol Cell Endocrinol. 2023 Dec 1;578:112066. doi: 10.1016/j.mce.2023.112066. Epub 2023 Sep 9. Mol Cell Endocrinol. 2023. PMID: 37690473 Free PMC article.
References
-
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006; 147:S56–S69. - PubMed
-
- Huo W, Xia W, Wan Y, Zhang B, Zhou A, Zhang Y, Huang K, Zhu Y, Wu C, Peng Y, Jiang M, Hu J et al. Maternal urinary bisphenol A levels and infant low birth weight: a nested case-control study of the health baby cohort in China. Environ Int 2015; 85:96–103. - PubMed
-
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002; 17:2839–2841. - PubMed
-
- Lee J, Choi K, Park J, Moon HB, Choi G, Lee JJ, Suh E, Kim HJ, Eun SH, Kim GH, Cho GJ, Kim SK et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci Total Environ 2018; 626:1494–1501. - PubMed

