Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis
- PMID: 35220429
- PMCID: PMC9071231
- DOI: 10.1093/humrep/deac035
Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis
Abstract
Study question: Is it safe to perform controlled ovarian stimulation (COS) for fertility preservation before starting anticancer therapies or ART after treatments in young breast cancer patients?
Summary answer: Performing COS before, or ART following anticancer treatment in young women with breast cancer does not seem to be associated with detrimental prognostic effect in terms of breast cancer recurrence, mortality or event-free survival (EFS).
What is known already: COS for oocyte/embryo cryopreservation before starting chemotherapy is standard of care for young women with breast cancer wishing to preserve fertility. However, some oncologists remain concerned on the safety of COS, particularly in patients with hormone-sensitive tumors, even when associated with aromatase inhibitors. Moreover, limited evidence exists on the safety of ART in breast cancer survivors for achieving pregnancy after the completion of anticancer treatments.
Study design, size, duration: The present systematic review and meta-analysis was carried out by three blinded investigators using the keywords 'breast cancer' and 'fertility preservation'; keywords were combined with Boolean operators. Eligible studies were identified by a systematic literature search of Medline, Web of Science, Embase and Cochrane library with no language or date restriction up to 30 June 2021.
Participants/materials, setting, methods: To be included in this meta-analysis, eligible studies had to be case-control or cohort studies comparing survival outcomes of women who underwent COS or ART before or after breast cancer treatments compared to breast cancer patients not exposed to these strategies. Survival outcomes of interest were cancer recurrence rate, relapse rate, overall survival and number of deaths. Adjusted relative risk (RR) and hazard ratio (HR) with 95% CI were extracted. When the number of events for each group were available but the above measures were not reported, HRs were estimated using the Watkins and Bennett method. We excluded case reports or case series with <10 patients and studies without a control group of breast cancer patients who did not pursue COS or ART. Quality of data and risk of bias were assessed using the Newcastle-Ottawa Assessment Scale.
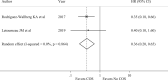
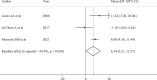
Main results and the role of chance: A total of 1835 records were retrieved. After excluding ineligible publications, 15 studies were finally included in the present meta-analysis (n = 4643). Among them, 11 reported the outcomes of breast cancer patients who underwent COS for fertility preservation before starting chemotherapy, and 4 the safety of ART following anticancer treatment completion. Compared to women who did not receive fertility preservation at diagnosis (n = 2386), those who underwent COS (n = 1594) had reduced risk of recurrence (RR 0.58, 95% CI 0.46-0.73) and mortality (RR 0.54, 95% CI 0.38-0.76). No detrimental effect of COS on EFS was observed (HR 0.76, 95% CI 0.55-1.06). A similar trend of better outcomes in terms of EFS was observed in women with hormone-receptor-positive disease who underwent COS (HR 0.36, 95% CI 0.20-0.65). A reduced risk of recurrence was also observed in patients undergoing COS before neoadjuvant chemotherapy (RR 0.22, 95% CI 0.06-0.80). Compared to women not exposed to ART following completion of anticancer treatments (n = 540), those exposed to ART (n = 123) showed a tendency for better outcomes in terms of recurrence ratio (RR 0.34, 95% CI 0.17-0.70) and EFS (HR 0.43, 95% CI 0.17-1.11).
Limitations, reasons for caution: This meta-analysis is based on abstracted data and most of the studies included are retrospective cohort studies. Not all studies had matching criteria between the study population and the controls, and these criteria often differed between the studies. Moreover, rate of recurrence is reported as a punctual event and it is not possible to establish when recurrences occurred and whether follow-up, which was shorter than 5 years in some of the included studies, is adequate to capture late recurrences.
Wider implications of the findings: Our results demonstrate that performing COS at diagnosis or ART following treatment completion does not seem to be associated with detrimental prognostic effect in young women with breast cancer, including among patients with hormone receptor-positive disease and those receiving neoadjuvant chemotherapy.
Study funding/competing interest(s): Partially supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC; grant number MFAG 2020 ID 24698) and the Italian Ministry of Health-5 × 1000 funds 2017 (no grant number). M.L. acted as consultant for Roche, Pfizer, Novartis, Lilly, AstraZeneca, MSD, Exact Sciences, Gilead, Seagen and received speaker honoraria from Roche, Pfizer, Novartis, Lilly, Ipsen, Takeda, Libbs, Knight, Sandoz outside the submitted work. F.S. acted as consultant for Novartis, MSD, Sun Pharma, Philogen and Pierre Fabre and received speaker honoraria from Roche, Novartis, BMS, MSD, Merck, Sun Pharma, Sanofi and Pierre Fabre outside the submitted work. I.D. has acted as a consultant for Roche, has received research grants from Roche and Ferring, has received reagents for academic clinical trial from Roche diagnostics, speaker's fees from Novartis, and support for congresses from Theramex and Ferring outside the submitted work. L.D.M. reported honoraria from Roche, Novartis, Eli Lilly, MSD, Pfizer, Ipsen, Novartis and had an advisory role for Roche, Eli Lilly, Novartis, MSD, Genomic Health, Pierre Fabre, Daiichi Sankyo, Seagen, AstraZeneca, Eisai outside the submitted work. The other authors declare no conflict of interest. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Registration number: N/A.
Keywords: ART; COS; assisted reproductive technologies; breast cancer; controlled ovarian stimulation; fertility preservation; young women.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures




References
-
- Azim AA, Costantini-Ferrando M, Oktay K.. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol 2008;26:2630–2635. - PubMed
-
- Ben-Haroush A, Farhi J, Ben-Aharon I, Sapir O, Pinkas H, Fisch B.. High yield of oocytes without an increase in circulating estradiol levels in breast cancer patients treated with follicle-stimulating hormone and aromatase inhibitor in standard gonadotropin-releasing hormone analogue protocols. Isr Med Assoc J 2011;13:753–756. - PubMed
-
- Blondeaux E, Massarotti C, Fontana V, Poggio F, Arecco L, Fregatti P, Bighin C, Giannubilo I, Ruelle T, Razeti MG. et al. The PREgnancy and FERtility (PREFER) Study Investigating the need for ovarian function and/or fertility preservation strategies in premenopausal women with early breast cancer. Front Oncol 2021;11:690320. - PMC - PubMed
-
- Bonardi B, Massarotti C, Bruzzone M, Goldrat O, Mangili G, Anserini P, Spinaci S, Arecco L, Del Mastro L, Ceppi M. et al. Efficacy and safety of controlled ovarian stimulation with or without letrozole co-administration for fertility preservation: a systematic review and meta-analysis. Front Oncol 2020;10:574669. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

