Can hysterosalpingo-foam sonography replace hysterosalpingography as first-choice tubal patency test? A randomized non-inferiority trial
- PMID: 35220432
- PMCID: PMC9071226
- DOI: 10.1093/humrep/deac034
Can hysterosalpingo-foam sonography replace hysterosalpingography as first-choice tubal patency test? A randomized non-inferiority trial
Abstract
Study question: Does hysterosalpingo-foam sonography (HyFoSy) lead to similar pregnancy outcomes, compared with hysterosalpingography (HSG), as first-choice tubal patency test in infertile couples?
Summary answer: HyFoSy and HSG produce similar findings in a majority of patients and clinical management based on the results of either HyFoSy or HSG, leads to comparable pregnancy outcomes. HyFoSy is experienced as significantly less painful.
What is known already: Traditionally, tubal patency testing during fertility work-up is performed by HSG. HyFoSy is an alternative imaging technique lacking ionizing radiation and iodinated contrast medium exposure which is less expensive than HSG. Globally, there is a shift towards the use of office-based diagnostic methods, such as HyFoSy.
Study design, size, duration: This multicentre, prospective, comparative study with a randomized design was conducted in 26 hospitals in The Netherlands. Participating women underwent both HyFoSy and HSG in randomized order. In case of discordant results, women were randomly allocated to either a management strategy based on HyFoSy or one based on HSG.
Participants/materials, setting, methods: We included infertile women between 18 and 41 years old who were scheduled for tubal patency testing during their fertility work-up. Women with anovulatory cycles not responding to ovulation induction, endometriosis, severe male infertility or a known iodine contrast allergy were excluded. The primary outcome for the comparison of the HyFoSy- and HSG-based strategies was ongoing pregnancy leading to live birth within 12 months after inclusion in an intention-to-treat analysis.
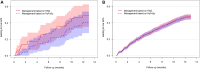
Main results and the role of chance: Between May 2015 and January 2019, 1026 women underwent HyFoSy and HSG. HyFoSy was inconclusive in 97 of them (9.5%), HSG was inconclusive in 30 (2.9%) and both were inconclusive in 9 (0.9%). In 747 women (73%) conclusive tests results were concordant. Of the 143/1026 (14%) with discordant results, 105 were randomized to clinical management based on the results of either HyFoSy or HSG. In this group, 22 of the 54 women (41%) allocated to management based on HyFoSy and 25 of 51 women (49%) allocated to management based on HSG had an ongoing pregnancy leading to live birth (Difference -8%; 95% CI: -27% to 10%). In total, clinical management based on the results of HyFoSy was estimated to lead to a live birth in 474 of 1026 women (46%) versus 486 of 1026 (47%) for management based on HSG (Difference -1.2%; 95% CI: -3.4% to 1.5%). Given the pre-defined margin of -2%, statistically significant non-inferiority of HyFoSy relative to HSG could not be demonstrated (P = 0.27). The mean pain score for HyFoSy on the 1-10 Visual Analogue Scale (VAS) was 3.1 (SD 2.2) and the mean VAS pain score for HSG was 5.4 (SD 2.5; P for difference < 0.001).
Limitations, reasons for caution: Since all women underwent both tubal patency tests, no conclusions on a direct therapeutic effect of tubal flushing could be drawn.
Wider implications of the findings: HyFoSy or HSG produce similar tubal pathology findings in a majority of infertile couples and, where they differ, a difference in findings does not lead to substantial difference in pregnancy outcome, while HyFoSy is associated with significantly less pain.
Study funding/competing interest(s): The FOAM study was an investigator-initiated study funded by ZonMw, The Netherlands organization for Health Research and Development (project number 837001504). ZonMw funded the whole project. IQ Medical Ventures provided the ExEm-foam® kits free of charge. The funders had no role in study design, collection, analysis and interpretation of the data. K.D. reports travel and speaker fees from Guerbet. F.J.M.B. reports personal fees as a member of the external advisory board for Merck Serono, The Netherlands, and a research support grant from Merck Serono, outside the submitted work. C.B.L. reports speakers' fee from Ferring in the past, and his department receives research grants from Ferring, Merck and Guerbet. J.S. reports a research agreement with Takeda on MR of motility outside the submitted work. M.V.W. reports leading The Netherlands Satellite of the Cochrane Gynaecology and Fertility Group. B.W.J.M. is supported by an NHMRC Investigator grant (GNT1176437). B.W.J.M. reports consultancy for Guerbet and research funding from Merck and Guerbet. V.M. reports non-financial support from IQ medicals ventures, during the conduct of the study; grants and personal fees from Guerbet, outside the submitted work. The other authors do not report conflicts of interest.
Trial registration number: NTR4746/NL4587 (https://www.trialregister.nl).
Trial registration date: 19 August 2014.
Date of first patient’s enrolment: 7 May 2015.
Keywords: effectiveness; fertility work-up; hysterosalpingo-foam sonography; hysterosalpingography; live birth; ongoing pregnancy; tubal patency test; tubal pathology.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures

Comment in
-
Reply: 'One-stop shop' ultrasound evaluation of an infertile patient: doing less is no longer an option.Hum Reprod. 2022 Jul 30;37(8):1953-1954. doi: 10.1093/humrep/deac133. Hum Reprod. 2022. PMID: 35713336 Free PMC article. No abstract available.
-
'One-stop shop' ultrasound evaluation of an infertile patient: doing less is no longer an option.Hum Reprod. 2022 Jul 30;37(8):1952-1953. doi: 10.1093/humrep/deac132. Hum Reprod. 2022. PMID: 35713337 No abstract available.
References
-
- ACOG. Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstet Gynecol 2019;133:e377–e384. - PubMed
-
- AHRQ. AHRQ Methods for Effective Health Care Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US), 2008. - PubMed
-
- Bossuyt PM, Lijmer JG, Mol BW.. Randomised comparisons of medical tests: sometimes invalid, not always efficient. Lancet 2000;356:1844–1847. - PubMed
-
- Chauhan MB, Lakra P, Jyotsna D, Nanda S, Malhotra V.. Pain relief during hysterosalpingography: role of intracervical block. Arch Gynecol Obstet 2013;287:155–159. - PubMed
-
- Collins JA, Burrows EA, Wilan AR.. The prognosis for live birth among untreated infertile couples. Fertil Steril 1995;64:22–28. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

