Direct effect of lipopolysaccharide and histamine on permeability of the rumen epithelium of steers ex vivo
- PMID: 35220439
- PMCID: PMC8903145
- DOI: 10.1093/jas/skac005
Direct effect of lipopolysaccharide and histamine on permeability of the rumen epithelium of steers ex vivo
Abstract
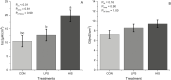
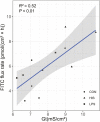
Disruption of the ruminal epithelium barrier occurs during subacute ruminal acidosis due to low pH, hyper-osmolality, and increased concentrations of lipopolysaccharide and histamine in ruminal fluid. However, the individual roles of lipopolysaccharide and histamine in the process of ruminal epithelium barriers disruption are not clear. The objective of the present investigation was to evaluate the direct effect of lipopolysaccharide and histamine on the barrier function of the ruminal epithelium. Compared with control (CON), histamine (HIS, 20 μM) increased the short-circuit current (Isc; 88.2%, P < 0.01), transepithelial conductance (Gt; 29.7%, P = 0.056), and the permeability of fluorescein 5(6)-isothiocyanate (FITC) (1.04-fold, P < 0.01) of ruminal epithelium. The apparent permeability of LPS was 1.81-fold higher than HIS (P < 0.01). The mRNA abundance of OCLN in ruminal epithelium was decreased by HIS (1.1-fold, P = 0.047). The results of the present study suggested that mucosal histamine plays a direct role in the disruption of ruminal epithelium barrier function, whereas lipopolysaccharide (at a pH of 7.4) has no effect on the permeability of rumen tissues ex vivo.
Keywords: gastrointestinal tract; permeability; subacute rumen acidosis.
Plain language summary
Lipopolysaccharide and histamine are common chemicals in rumen when the ruminant animal takes too much concentrate. We wandered whether these two chemicals have direct effects on the rumen tissues. Using Ussing chamber, we found that histamine could directly improve the permeability of rumen barrier.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Feeding barley grain-rich diets altered electrophysiological properties and permeability of the ruminal wall in a goat model.J Dairy Sci. 2013 Apr;96(4):2293-2302. doi: 10.3168/jds.2012-6187. Epub 2013 Feb 10. J Dairy Sci. 2013. PMID: 23403198
-
Effects of subacute ruminal acidosis on colon epithelial morphological structure, permeability, and expression of key tight junction proteins in dairy goats.J Dairy Sci. 2021 Apr;104(4):4260-4270. doi: 10.3168/jds.2020-18738. Epub 2021 Jan 21. J Dairy Sci. 2021. PMID: 33485680
-
Key role of short-chain fatty acids in epithelial barrier failure during ruminal acidosis.J Dairy Sci. 2017 Aug;100(8):6662-6675. doi: 10.3168/jds.2016-12262. Epub 2017 May 24. J Dairy Sci. 2017. PMID: 28551186
-
Ruminal acidosis, bacterial changes, and lipopolysaccharides.J Anim Sci. 2020 Aug 1;98(8):skaa248. doi: 10.1093/jas/skaa248. J Anim Sci. 2020. PMID: 32761212 Free PMC article. Review.
-
Symposium review: The importance of the ruminal epithelial barrier for a healthy and productive cow.J Dairy Sci. 2019 Feb;102(2):1866-1882. doi: 10.3168/jds.2018-15243. Epub 2018 Dec 20. J Dairy Sci. 2019. PMID: 30580938 Review.
Cited by
-
The Role of Rumen Microbiota and Its Metabolites in Subacute Ruminal Acidosis (SARA)-Induced Inflammatory Diseases of Ruminants.Microorganisms. 2022 Jul 25;10(8):1495. doi: 10.3390/microorganisms10081495. Microorganisms. 2022. PMID: 35893553 Free PMC article. Review.
-
Integrated microbiota-host-metabolome approaches reveal adaptive ruminal changes to prolonged high-grain feeding and phytogenic supplementation in cattle.FEMS Microbiol Ecol. 2024 Jan 24;100(2):fiae006. doi: 10.1093/femsec/fiae006. FEMS Microbiol Ecol. 2024. PMID: 38281064 Free PMC article.
-
Transcriptomics insights into glutamine on repairing of histamine-induced Yak rumen epithelial cells barrier damage in vitro.BMC Genomics. 2025 Feb 25;26(1):195. doi: 10.1186/s12864-025-11383-6. BMC Genomics. 2025. PMID: 40000997 Free PMC article.
-
Role of the Rumen Epithelium and Associated Changes Under High-Concentrate Diets.Int J Mol Sci. 2025 Mar 13;26(6):2573. doi: 10.3390/ijms26062573. Int J Mol Sci. 2025. PMID: 40141216 Free PMC article. Review.
-
Enzymatically prepared alginate oligosaccharides improve broiler chicken growth performance by modulating the gut microbiota and growth hormone signals.J Anim Sci Biotechnol. 2023 Jul 3;14(1):96. doi: 10.1186/s40104-023-00887-4. J Anim Sci Biotechnol. 2023. PMID: 37394467 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

