Comparison of Machine Learning Methods Using Spectralis OCT for Diagnosis and Disability Progression Prognosis in Multiple Sclerosis
- PMID: 35220529
- PMCID: PMC9001622
- DOI: 10.1007/s10439-022-02930-3
Comparison of Machine Learning Methods Using Spectralis OCT for Diagnosis and Disability Progression Prognosis in Multiple Sclerosis
Abstract
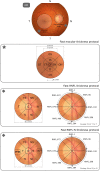
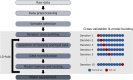
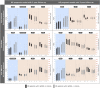
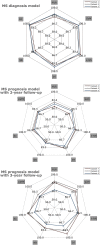
Machine learning approaches in diagnosis and prognosis of multiple sclerosis (MS) were analysed using retinal nerve fiber layer (RNFL) thickness, measured by optical coherence tomography (OCT). A cross-sectional study (72 MS patients and 30 healthy controls) was used for diagnosis. These 72 MS patients were involved in a 10-year longitudinal follow-up study for prognostic purposes. Structural measurements of RNFL thickness were performed using different Spectralis OCT protocols: fast macular thickness protocol to measure macular RNFL, and fast RNFL thickness protocol and fast RNFL-N thickness protocol to measure peripapillary RNFL. Binary classifiers such as multiple linear regression (MLR), support vector machines (SVM), decision tree (DT), k-nearest neighbours (k-NN), Naïve Bayes (NB), ensemble classifier (EC) and long short-term memory (LSTM) recurrent neural network were tested. For MS diagnosis, the best acquisition protocol was fast macular thickness protocol using k-NN (accuracy: 95.8%; sensitivity: 94.4%; specificity: 97.2%; precision: 97.1%; AUC: 0.958). For MS prognosis, our model with a 3-year follow up to predict disability progression 8 years later was the best predictive model. DT performed best for fast macular thickness protocol (accuracy: 91.3%; sensitivity: 90.0%; specificity: 92.5%; precision: 92.3%; AUC: 0.913) and SVM for fast RNFL-N thickness protocol (accuracy: 91.3%; sensitivity: 87.5%; specificity: 95.0%; precision: 94.6%; AUC: 0.913). This work concludes that measurements of RNFL thickness obtained with Spectralis OCT have a good ability to diagnose MS and to predict disability progression in MS patients. This machine learning approach would help clinicians to have valuable information.
Keywords: Machine learning; Multiple sclerosis; Optical coherence tomography; Retinal nerve fiber layer.
© 2022. The Author(s).
Conflict of interest statement
The authors state that there are no conflicts of interest.
Figures


Similar articles
-
Machine learning in diagnosis and disability prediction of multiple sclerosis using optical coherence tomography.Comput Biol Med. 2021 Jun;133:104416. doi: 10.1016/j.compbiomed.2021.104416. Epub 2021 Apr 26. Comput Biol Med. 2021. PMID: 33946022
-
Swept source optical coherence tomography to early detect multiple sclerosis disease. The use of machine learning techniques.PLoS One. 2019 May 6;14(5):e0216410. doi: 10.1371/journal.pone.0216410. eCollection 2019. PLoS One. 2019. PMID: 31059539 Free PMC article. Clinical Trial.
-
Retinal imaging by laser polarimetry and optical coherence tomography evidence of axonal degeneration in multiple sclerosis.Arch Neurol. 2008 Jul;65(7):924-8. doi: 10.1001/archneur.65.7.924. Arch Neurol. 2008. PMID: 18625859
-
The role of machine learning in developing non-magnetic resonance imaging based biomarkers for multiple sclerosis: a systematic review.BMC Med Inform Decis Mak. 2022 Sep 15;22(1):242. doi: 10.1186/s12911-022-01985-5. BMC Med Inform Decis Mak. 2022. PMID: 36109726 Free PMC article.
-
An analytical review on the use of artificial intelligence and machine learning in diagnosis, prediction, and risk factor analysis of multiple sclerosis.Mult Scler Relat Disord. 2024 Sep;89:105761. doi: 10.1016/j.msard.2024.105761. Epub 2024 Jul 16. Mult Scler Relat Disord. 2024. PMID: 39018642 Review.
Cited by
-
Differential Study of Retinal Thicknesses in the Eyes of Alzheimer's Patients, Multiple Sclerosis Patients and Healthy Subjects.Biomedicines. 2023 Nov 24;11(12):3126. doi: 10.3390/biomedicines11123126. Biomedicines. 2023. PMID: 38137347 Free PMC article.
-
Optical Coherence Tomography and Optical Coherence Tomography with Angiography in Multiple Sclerosis.Healthcare (Basel). 2022 Jul 25;10(8):1386. doi: 10.3390/healthcare10081386. Healthcare (Basel). 2022. PMID: 35893208 Free PMC article. Review.
-
ARTIFICIAL INTELLIGENCE FOR OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY-BASED DISEASE ACTIVITY PREDICTION IN AGE-RELATED MACULAR DEGENERATION.Retina. 2024 Mar 1;44(3):465-474. doi: 10.1097/IAE.0000000000003977. Retina. 2024. PMID: 37988102 Free PMC article.
-
[Retinal OCT biomarkers and neurodegenerative diseases of the central nervous system beyond Alzheimer's disease].Ophthalmologie. 2024 Feb;121(2):93-104. doi: 10.1007/s00347-023-01974-7. Epub 2024 Jan 23. Ophthalmologie. 2024. PMID: 38263475 Review. German.
-
Diagnosis of multiple sclerosis using optical coherence tomography supported by explainable artificial intelligence.Eye (Lond). 2024 Jun;38(8):1502-1508. doi: 10.1038/s41433-024-02933-5. Epub 2024 Jan 31. Eye (Lond). 2024. PMID: 38297153 Free PMC article.
References
-
- Birkeldh U, Manouchehrinia A, Hietala MA, Hillert J, Olsson T, Piehl F, Kockum IS, Brundin L, Zahavi O, Wahlberg-Ramsay M, Brautaset R, Nilsson M. The temporal retinal nerve fiber layer thickness is the most important optical coherence tomography estimate in multiple sclerosis. Front. Neurol. 2017;8:8. doi: 10.3389/fneur.2017.00675. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

