Hematopoietic stem cell transplantation ameliorates maternal diabetes-mediated gastrointestinal symptoms and autism-like behavior in mouse offspring
- PMID: 35220596
- PMCID: PMC9307016
- DOI: 10.1111/nyas.14766
Hematopoietic stem cell transplantation ameliorates maternal diabetes-mediated gastrointestinal symptoms and autism-like behavior in mouse offspring
Abstract
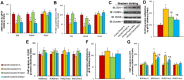
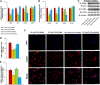
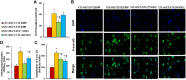
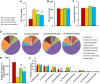
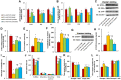
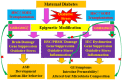
Epidemiological studies have shown that maternal diabetes is associated with autism spectrum disorder development, although the detailed mechanism remains unclear. We have previously found that maternal diabetes induces persistent epigenetic changes and gene suppression in neurons, subsequently triggering autism-like behavior (ALB). In this study, we investigated the potential role and effect of hematopoietic stem cells (HSCs) on maternal diabetes-mediated gastrointestinal (GI) dysfunction and ALB in a mouse model. We show in vitro that transient hyperglycemia induced persistent epigenetic changes and gene suppression of tight junction proteins. In vivo, maternal diabetes-mediated oxidative stress induced gene suppression and inflammation in both peripheral blood mononuclear cells and intestine epithelial cells, subsequently triggering GI dysfunction with increased intestinal permeability and altered microbiota compositions, as well as suppressed gene expression in neurons and subsequent ALB in offspring; HSC transplantation (HSCT) ameliorates this effect by systematically reversing maternal diabetes-mediated oxidative stress. We conclude that HSCT can ameliorate maternal diabetes-mediated GI symptoms and autism-like behavior in mouse offspring.
Keywords: autism; autism spectrum disorder; gastrointestinal symptoms; hematopoietic stem cells; intestinal epithelial cells; maternal diabetes.
© 2022 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals LLC on behalf of New York Academy of Sciences.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

