Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH)
- PMID: 35220659
- PMCID: PMC9311407
- DOI: 10.1111/liv.15207
Effect of rifaximin on infections, acute-on-chronic liver failure and mortality in alcoholic hepatitis: A pilot study (RIFA-AH)
Abstract
Background & aims: Alcoholic hepatitis (AH) is associated with a high incidence of infection and mortality. Rifaximin reduces bacterial overgrowth and translocation. We aimed to study whether the administration of rifaximin as an adjuvant treatment to corticosteroids decreases the number of bacterial infections at 90 days in patients with severe AH compared to a control cohort.
Methods: This was a multicentre, open, comparative pilot study of the addition of rifaximin (1200 mg/day/90 days) to the standard treatment for severe AH. The results were compared with a carefully matched historical cohort of patients treated with standard therapy and matching by age and model of end-stage liver disease (MELD). We evaluated bacterial infections, liver-related complications, mortality and liver function tests after 90 days.
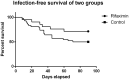
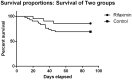
Results: Twenty-one and 42 patients were included in the rifaximin and control groups respectively. No significant baseline differences were found between groups. The mean number of infections per patient was 0.29 and 0.62 in the rifaximin and control groups, respectively (p = .049), with a lower incidence of acute-on-chronic liver failure (ACLF) linked to infections within the treatment group. Liver-related complications were lower within the rifaximin group (0.43 vs. 1.26 complications/patient respectively) (p = .01). Mortality was lower in the treated versus the control groups (14.2% vs. 30.9, p = .15) without significant differences. No serious adverse events were associated with rifaximin treatment.
Conclusions: Rifaximin is safe in severe AH with a significant reduction in clinical complications. A lower number of infections and a trend towards a lower ACLF and mortality favours its use in these patients.
Keywords: acute-on-chronic liver failure; alcohol-related liver disease; bacterial infection; cirrhosis; rifaximin; severe alcoholic hepatitis.
© 2022 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
BS has been consulting for the Ferring Research Institute, Gelesis, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals and Takeda. BS’s institution UC San Diego has received research support from Axial Biotherapeutics, BiomX, CymaBay Therapeutics, NGM Biopharmaceuticals, Prodigy Biotech and Synlogic Operating Company. BS is a founder of Nterica Bio. UC San Diego has registered several patents with BS as an inventor related to this work. DS has performed consultancy, delivered paid lectures and is taking part in two investigator‐initiated studies (REEFSYS and EMITTIC) funded by Alfasigma/Norgine, who manufacture rifaximin.
Figures


Comment in
-
Value of pilot studies in alcohol-associated hepatitis.Liver Int. 2022 Jul;42(7):1697. doi: 10.1111/liv.15291. Epub 2022 May 23. Liver Int. 2022. PMID: 35567756 No abstract available.
-
Rifaximin for severe alcoholic hepatitis-Attractive but unfructuous.Liver Int. 2022 Jul;42(7):1698-1699. doi: 10.1111/liv.15294. Epub 2022 May 20. Liver Int. 2022. PMID: 35567764 No abstract available.
References
-
- Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta‐analysis of randomized trials. Liver Int. 2016;36:721‐728. - PubMed
-
- Moreau R. The pathogenesis of ACLF: the inflammatory response and immune function. Semin Liver Dis. 2016;36:133‐140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

