Aptamers for Viral Detection and Inhibition
- PMID: 35220716
- PMCID: PMC8905934
- DOI: 10.1021/acsinfecdis.1c00546
Aptamers for Viral Detection and Inhibition
Abstract
Recent times have experienced more than ever the impact of viral infections in humans. Viral infections are known to cause diseases not only in humans but also in plants and animals. Here, we have compiled the literature review of aptamers selected and used for detection and inhibition of viral infections in all three categories: humans, animals, and plants. This review gives an in-depth introduction to aptamers, different types of aptamer selection (SELEX) methodologies, the benefits of using aptamers over commonly used antibody-based strategies, and the structural and functional mechanism of aptasensors for viral detection and therapy. The review is organized based on the different characterization and read-out tools used to detect virus-aptasensor interactions with a detailed index of existing virus-targeting aptamers. Along with addressing recent developments, we also discuss a way forward with aptamers for DNA nanotechnology-based detection and treatment of viral diseases. Overall, this review will serve as a comprehensive resource for aptamer-based strategies in viral diagnostics and treatment.
Keywords: DNA nanostructures; aptamers; aptasensors; inhibition; sensing; viruses.
Figures



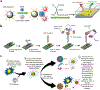


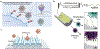


Similar articles
-
Aptamers: The Powerful Molecular Tools for Virus Detection.Chem Asian J. 2021 Jun 1;16(11):1298-1306. doi: 10.1002/asia.202100242. Epub 2021 May 1. Chem Asian J. 2021. PMID: 33851522 Review.
-
Application of aptamer-based viral detection in animals.Pol J Vet Sci. 2023 Sep 20;26(3):521-529. doi: 10.24425/pjvs.2023.145056. Pol J Vet Sci. 2023. PMID: 37727988 Review.
-
Application of Electrochemical Aptasensors toward Clinical Diagnostics, Food, and Environmental Monitoring: Review.Sensors (Basel). 2019 Dec 10;19(24):5435. doi: 10.3390/s19245435. Sensors (Basel). 2019. PMID: 31835479 Free PMC article. Review.
-
Application of Aptamers in Virus Detection and Antiviral Therapy.Front Microbiol. 2019 Jul 3;10:1462. doi: 10.3389/fmicb.2019.01462. eCollection 2019. Front Microbiol. 2019. PMID: 31333603 Free PMC article. Review.
-
Advances in aptasensor technology.Adv Clin Chem. 2020;99:237-279. doi: 10.1016/bs.acc.2020.02.010. Epub 2020 Mar 12. Adv Clin Chem. 2020. PMID: 32951638 Review.
Cited by
-
New Virus Diagnostic Approaches to Ensuring the Ongoing Plant Biosecurity of Aotearoa New Zealand.Viruses. 2023 Feb 1;15(2):418. doi: 10.3390/v15020418. Viruses. 2023. PMID: 36851632 Free PMC article. Review.
-
Overcoming Limited Access to Virus Infection Rapid Testing: Development of a Lateral Flow Test for SARS-CoV-2 with Locally Available Resources.Biosensors (Basel). 2024 Aug 27;14(9):416. doi: 10.3390/bios14090416. Biosensors (Basel). 2024. PMID: 39329791 Free PMC article.
-
Aptamer-functionalized field-effect transistor biosensors for disease diagnosis and environmental monitoring.Exploration (Beijing). 2023 May 11;3(3):20210027. doi: 10.1002/EXP.20210027. eCollection 2023 Jun. Exploration (Beijing). 2023. PMID: 37933385 Free PMC article. Review.
-
DNA-Based Nanomaterials as Drug Delivery Platforms for Increasing the Effect of Drugs in Tumors.Cancers (Basel). 2023 Apr 5;15(7):2151. doi: 10.3390/cancers15072151. Cancers (Basel). 2023. PMID: 37046816 Free PMC article. Review.
-
Generation of RNA aptamers against chikungunya virus E2 envelope protein.J Virol. 2025 Mar 18;99(3):e0209524. doi: 10.1128/jvi.02095-24. Epub 2025 Feb 10. J Virol. 2025. PMID: 39927773 Free PMC article.
References
-
- Tobin NH; Campbell AJP; Zerr DM; Melvin AJ, Life-Threatening Viral Diseases and Their Treatment. In Pediatric Critical Care, 2011, 1324–1335.
-
- Ménard-Moyon C; Bianco A; Kalantar-Zadeh K, Two-Dimensional Material-Based Biosensors for Virus Detection. ACS Sensors 2020, 5 (12), 3739–3769. - PubMed
-
- Kevadiya BD; Machhi J; Herskovitz J; Oleynikov MD; Blomberg WR; Bajwa N; Soni D; Das S; Hasan M; Patel M; Senan AM; Gorantla S; McMillan J; Edagwa B; Eisenberg R; Gurumurthy CB; Reid SPM; Punyadeera C; Chang L; Gendelman HE, Diagnostics for SARS-CoV-2 infections. Nat. Mater 2021, 20 (5), 593–605. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

