Estimating the percentage of patients who might benefit from proton beam therapy instead of X-ray radiotherapy
- PMID: 35220723
- PMCID: PMC10993980
- DOI: 10.1259/bjr.20211175
Estimating the percentage of patients who might benefit from proton beam therapy instead of X-ray radiotherapy
Abstract
Objectives: High-energy Proton Beam Therapy (PBT) commenced in England in 2018 and NHS England commissions PBT for 1.5% of patients receiving radical radiotherapy. We sought expert opinion on the level of provision.
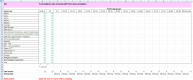
Methods: Invitations were sent to 41 colleagues working in PBT, most at one UK centre, to contribute by completing a spreadsheet. 39 responded: 23 (59%) completed the spreadsheet; 16 (41%) declined, arguing that clinical outcome data are lacking, but joined six additional site-specialist oncologists for two consensus meetings. The spreadsheet was pre-populated with incidence data from Cancer Research UK and radiotherapy use data from the National Cancer Registration and Analysis Service. 'Mechanisms of Benefit' of reduced growth impairment, reduced toxicity, dose escalation and reduced second cancer risk were examined.
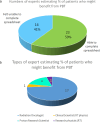
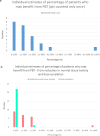
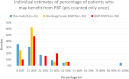
Results: The most reliable figure for percentage of radical radiotherapy patients likely to benefit from PBT was that agreed by 95% of the 23 respondents at 4.3%, slightly larger than current provision. The median was 15% (range 4-92%) and consensus median 13%. The biggest estimated potential benefit was from reducing toxicity, median benefit to 15% (range 4-92%), followed by dose escalation median 3% (range 0 to 47%); consensus values were 12 and 3%. Reduced growth impairment and reduced second cancer risk were calculated to benefit 0.5% and 0.1%.
Conclusions: The most secure estimate of percentage benefit was 4.3% but insufficient clinical outcome data exist for confident estimates. The study supports the NHS approach of using the evidence base and developing it through randomised trials, non-randomised studies and outcomes tracking.
Advances in knowledge: Less is known about the percentage of patients who may benefit from PBT than is generally acknowledged. Expert opinion varies widely. Insufficient clinical outcome data exist to provide robust estimates. Considerable further work is needed to address this, including international collaboration; much is already underway but will take time to provide mature data.
Figures

References
-
- Langendijk JA . Current status of particle therapy in the netherlands . Radiotherapy and Oncology 2016. ; 118: S65 . doi: 10.1016/S0167-8140(16)30132-3 - DOI

