Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis
- PMID: 35220887
- PMCID: PMC8890384
- DOI: 10.1080/19490976.2022.2038852
Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis
Abstract
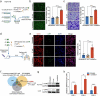
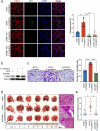
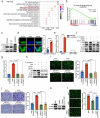
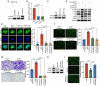
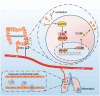
Metastasis is the leading cause of death for colorectal cancer (CRC) patients, and the spreading tumor cells adhesion to endothelial cells is a critical step for extravasation and further distant metastasis. Previous studies have documented the important roles of gut microbiota-host interactions in the CRC malignancy, and Fusobacterium nucleatum (F. nucleatum) was reported to increase proliferation and invasive activities of CRC cells. However, the potential functions and underlying mechanisms of F. nucleatum in the interactions between CRC cells and endothelial cells and subsequent extravasation remain unclear. Here, we uncovered that F. nucleatum enhanced the adhesion of CRC cells to endothelial cells, promoted extravasation and metastasis by inducing ICAM1 expression. Mechanistically, we identified that F. nucleatum induced a new pattern recognition receptor ALPK1 to activate NF-κB pathway, resulting in the upregulation of ICAM1. Interestingly, the abundance of F. nucleatum in tumor tissues of CRC patients was positively associated with the expression levels of ALPK1 and ICAM1. Moreover, high expression of ALPK1 or ICAM1 was significantly associated with a shorter overall survival time of CRC patients. This study provides a new insight into the role of gut microbiota in engaging into the distant metastasis of CRC cells.
Keywords: ALPK1; Gut microbes; ICAM1; adhesion; colorectal cancer metastasis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
