Clinical characteristics and patient outcomes of molecular subtypes of small cell lung cancer (SCLC)
- PMID: 35220975
- PMCID: PMC8883717
- DOI: 10.1186/s12957-022-02528-y
Clinical characteristics and patient outcomes of molecular subtypes of small cell lung cancer (SCLC)
Abstract
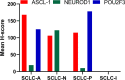
Background: Recent studies have shown that according to the expression levels of achaete-scute homolog 1 (ASCL1), neurogenic differentiation factor 1 (NEUROD1), and POU class 2 homeobox 3 (POU2F3), small cell lung cancer (SCLC) can be divided into four subtypes: SCLC-A (ASCL1-dominant), SCLC-N (NEUROD1-dominant), SCLC-P (POU2F3-dominant), and SCLC-I (triple negative or SCLC-inflamed). However, there are limited data on the clinical characteristics and prognosis of molecular subtypes of SCLC.
Methods: Immunohistochemistry (IHC) was used to detect the expression levels of ASCL1, NEUROD1, and POU2F3 in 53 patient samples of resectable SCLC. The subtype was defined by the differential expression of the transcription factors for ASCL1, NEUROD1, and POU2F3 or the low expression of all three factors with an inflamed gene signature (SCLC-A, SCLC-N, SCLC-P, and SCLC-I, respectively). The clinicopathological characteristics, immunological features (programmed death ligand 1 [PD-L1] expression and CD8+ tumor infiltrating lymphocyte [TIL] density), and patient outcomes of the four subtypes of SCLC were analyzed.
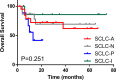
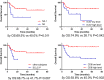
Results: Positive ASCL1, NEUROD1, and POU2F3 staining was detected in 43 (79.2%), 27 (51.0%), and 17 (32.1%) SCLC specimens by IHC. According to the results of IHC analysis, SCLC was divided into four subtypes: SCLC-A (39.6%), SCLC-N (28.3%), SCLC-P (17.0%), and SCLC-I (15.1%). The 5-year overall survival (OS) rates of these four subtypes were 61.9%, 69.3%, 41.7%, and 85.7%, respectively (P=0.251). There were significant differences in smoking status among different subtypes of SCLC (P= 0.031). However, we did not confirm the correlation between subtypes of SCLC and other clinicopathological factors or immune profiles. Cox multivariate analysis showed that N stage (P=0.025), CD8+ TILs (P=0.024), Ki-67 level (P=0.040), and SCLC-P (P=0.023) were independent prognostic factors for resectable SCLC.
Conclusions: Our IHC-based study validated the proposed classification of SCLC using the expression patterns of key transcriptional regulatory factors. We found that SCLC-P was associated with smokers and was one of the poor prognostic factors of limited-stage SCLC. In addition, no correlation was found between PD-L1 expression or CD8+ TIL density and SCLC subtypes.
Keywords: ASCL1; Molecular subtype; NEUROD1; POU2F3; Prognosis; Small cell lung cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Expression patterns and prognostic relevance of subtype-specific transcription factors in surgically resected small-cell lung cancer: an international multicenter study.J Pathol. 2022 Aug;257(5):674-686. doi: 10.1002/path.5922. Epub 2022 May 25. J Pathol. 2022. PMID: 35489038 Free PMC article.
-
Molecular subtypes, predictive markers and prognosis in small-cell lung carcinoma.J Clin Pathol. 2024 Dec 18;78(1):42-50. doi: 10.1136/jcp-2023-209109. J Clin Pathol. 2024. PMID: 37775262
-
SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization.J Thorac Oncol. 2020 Dec;15(12):1823-1835. doi: 10.1016/j.jtho.2020.09.009. Epub 2020 Oct 1. J Thorac Oncol. 2020. PMID: 33011388 Free PMC article.
-
Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data.Nat Rev Cancer. 2019 May;19(5):289-297. doi: 10.1038/s41568-019-0133-9. Nat Rev Cancer. 2019. PMID: 30926931 Free PMC article. Review.
-
Molecular Subtypes and Targeted Therapeutic Strategies in Small Cell Lung Cancer: Advances, Challenges, and Future Perspectives.Molecules. 2025 Apr 12;30(8):1731. doi: 10.3390/molecules30081731. Molecules. 2025. PMID: 40333678 Free PMC article. Review.
Cited by
-
UCHL1 acts as a potential oncogene and affects sensitivity of common anti-tumor drugs in lung adenocarcinoma.World J Surg Oncol. 2022 May 11;20(1):153. doi: 10.1186/s12957-022-02620-3. World J Surg Oncol. 2022. PMID: 35546675 Free PMC article.
-
Transitioning to a Personalized Approach in Molecularly Subtyped Small-Cell Lung Cancer (SCLC).Int J Mol Sci. 2024 Apr 10;25(8):4208. doi: 10.3390/ijms25084208. Int J Mol Sci. 2024. PMID: 38673793 Free PMC article. Review.
-
Identification of molecular subgroups and establishment of risk model based on the response to oxidative stress to predict overall survival of patients with lung adenocarcinoma.Eur J Med Res. 2023 Sep 9;28(1):333. doi: 10.1186/s40001-023-01290-5. Eur J Med Res. 2023. PMID: 37689745 Free PMC article.
-
Lung immune prognostic index (LIPI) as a prognostic factor in patients with extensive-stage small cell lung cancer treated with first-line chemoimmunotherapy.Clin Transl Oncol. 2025 Apr;27(4):1484-1492. doi: 10.1007/s12094-024-03690-3. Epub 2024 Sep 6. Clin Transl Oncol. 2025. PMID: 39240302
-
Redefining YAP1 in small cell lung cancer: shifting from a dominant subtype marker to a favorable prognostic indicator.Transl Lung Cancer Res. 2024 Aug 31;13(8):1768-1779. doi: 10.21037/tlcr-24-317. Epub 2024 Aug 17. Transl Lung Cancer Res. 2024. PMID: 39263025 Free PMC article.
References
-
- Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–1125. doi: 10.1016/S1470-2045(17)30318-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

