NAD+ in COVID-19 and viral infections
- PMID: 35221228
- PMCID: PMC8831132
- DOI: 10.1016/j.it.2022.02.001
NAD+ in COVID-19 and viral infections
Abstract
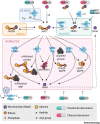
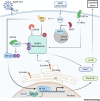
NAD+, as an emerging regulator of immune responses during viral infections, may be a promising therapeutic target for coronavirus disease 2019 (COVID-19). In this Opinion, we suggest that interventions that boost NAD+ levels might promote antiviral defense and suppress uncontrolled inflammation. We discuss the association between low NAD+ concentrations and risk factors for poor COVID-19 outcomes, including aging and common comorbidities. Mechanistically, we outline how viral infections can further deplete NAD+ and its roles in antiviral defense and inflammation. We also describe how coronaviruses can subvert NAD+-mediated actions via genes that remove NAD+ modifications and activate the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome. Finally, we explore ongoing approaches to boost NAD+ concentrations in the clinic to putatively increase antiviral responses while curtailing hyperinflammation.
Keywords: COVID-19; NAD(+); immune responses; inflammation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
D.A.S. is a founder, equity owner, advisor to, director of, board member of, consultant to, investor in and/or inventor on patents licensed to GlaxoSmithKline, Segterra, Animal Biosciences, AFAR, Cohbar, Galilei, Zymo Research, Immetas, EdenRoc Sciences and affiliates (Arc-Bio, Dovetail Genomics, Claret Bioscience, MetroBiotech, Astrea, Liberty Biosecurity and Delavie), Life Biosciences, and Levels Health. D.A.S. is an inventor on a patent application licensed to Elysium Health. More at
Figures
References
-
- Luk J., et al. Observations on mortality during the 1918 influenza pandemic. Clin. Infect. Dis. 2001;33:1375–1378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

