Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs
- PMID: 35221287
- PMCID: PMC11022858
- DOI: 10.1124/dmd.121.000417
Absorption, Distribution, Metabolism, and Excretion of US Food and Drug Administration-Approved Antisense Oligonucleotide Drugs
Abstract
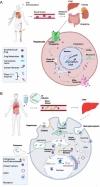
Absorption, distribution, metabolism, and excretion (ADME) are the key biologic processes for determination of a drug's pharmacokinetic parameters, which have direct impacts on efficacy and adverse drug reactions (ADRs). The chemical structures, dosage forms, and sites and routes of administration are the principal determinants of ADME profiles and consequent impacts on their efficacy and ADRs. Newly developed large molecule biologic antisense oligonucleotide (ASO) drugs have completely unique ADME that is not fully defined. ASO-based drugs are single-stranded synthetic antisense nucleic acids with diverse modes of drug actions from induction of mRNA degradation, exon skipping and restoration, and interactions with proteins. ASO drugs have a great potential to treat certain human diseases that have remained untreatable with small molecule-based drugs. The ADME of ASO drugs contributes to their unique set of ADRs and toxicity. In this review, to better understand their ADME, the 10 US Food and Drug Administration (FDA)-approved ASO drugs were selected: fomivirsen, pegaptanib, mipomersen, nusinersen, inotersen, defibrotide, eteplirsen, golodirsen, viltolarsen, and casimersen. A meta-analysis was conducted on their formulation, dosage, sites of administration, local and systematic distribution, metabolism, degradation, and excretion. Membrane permeabilization through endocytosis and nucleolytic degradation by endonucleases and exonucleases are major ADME features of the ASO drugs that differ from small-molecule drugs. The information summarized here provides comprehensive ADME characteristics of FDA-approved ASO drugs, leading to a better understanding of their therapeutic efficacy and their potential ADRs and toxicity. Numerous knowledge gaps, particularly on cellular uptake and subcellular trafficking and distribution, are identified, and future perspectives and directions are discussed. SIGNIFICANCE STATEMENT: Through a systematic analysis of the existing information of absorption, distribution, metabolism, and excretion (ADME) parameters for 10 US Food and Drug Administration (FDA)-approved antisense oligonucleotide (ASO) drugs, this review provides an overall view of the unique ADME characteristics of ASO drugs, which are distinct from small chemical drug ADME. This knowledge is useful for discovery and development of new ASO drugs as well as clinical use of current FDA-approved ASO drugs.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

