Different Electrochemical Behavior of Cationic Dopamine from Anionic Ascorbic Acid and DOPAC at CNT Yarn Microelectrodes
- PMID: 35221350
- PMCID: PMC8871592
- DOI: 10.1149/1945-7111/ac4d67
Different Electrochemical Behavior of Cationic Dopamine from Anionic Ascorbic Acid and DOPAC at CNT Yarn Microelectrodes
Abstract
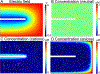
Carbon nanotube yarn microelectrodes (CNTYMEs) have micron-scale surface crevices that momentarily trap molecules. CNTYMEs improve selectivity among cationic catecholamines because secondary reactions are enhanced, but no anions have been studied. Here, we compared fast-scan cyclic voltammetry (FSCV) of dopamine and anionic interferents 3,4 dihydroxyphenylacetic acid (DOPAC) and L-ascorbic acid (AA) at CNTYMEs and carbon fiber microelectrodes (CFMEs). At CFMEs, dopamine current decreases with increasing FSCV repetition frequency at pH 7.4, whereas DOPAC and AA have increasing currents with increasing frequency, because of less repulsion at the negative holding potential. Both DOPAC and AA have side reactions after being oxidized, which are enhanced by trapping. At pH 4, the current increases for DOPAC and AA because they are not repelled. In addition, AA has a different oxidation pathway at pH 4, and an extra peak in the CV is enhanced by trapping effects at CNTYMEs. At pH 8.5, co-detection of dopamine in the presence of DOPAC and AA is enhanced at 100 Hz frequency because of differences in secondary peaks. Thus, the trapping effects at CNTYMEs affects anions differently than cations and secondary peaks can be used to identify dopamine in mixture of AA and DOPAC with FSCV.
Figures





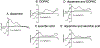

References
-
- Baur JE; Kristensen EW; May LJ; Wiedemann DJ; Wightman RM Fast-Scan Voltammetry of Biogenic Amines. Anal. Chem 1988, 60 (13), 1268–1272. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
