Xenobiotic metabolism and its physiological consequences in high-Antarctic Notothenioid fishes
- PMID: 35221461
- PMCID: PMC8818001
- DOI: 10.1007/s00300-021-02992-4
Xenobiotic metabolism and its physiological consequences in high-Antarctic Notothenioid fishes
Abstract
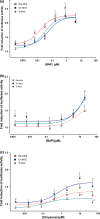
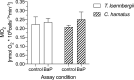
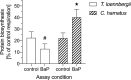
The Antarctic ecosystem is progressively exposed to anthropogenic contaminants, such as polycyclic aromatic hydrocarbons (PAHs). So far, it is largely unknown if PAHs leave a mark in the physiology of high-Antarctic fish. We approached this issue via two avenues: first, we examined the functional response of the aryl hydrocarbon receptor (Ahr), which is a molecular initiating event of many toxic effects of PAHs in biota. Chionodraco hamatus and Trematomus loennbergii served as representatives for high-Antarctic Notothenioids, and Atlantic cod, Gadus morhua as non-polar reference species. We sequenced and cloned the Ahr ligand binding domain (LBD) of the Notothenioids and deployed a GAL4-based luciferase reporter gene assay expressing the Ahr LBD. Benzo[a]pyrene (BaP), beta-naphthoflavone and chrysene were used as ligands for the reporter gene assay. Second, we investigated the energetic costs of Ahr activation in isolated liver cells of the Notothenioids during acute, non-cytotoxic BaP exposure. In the reporter assay, the Ahr LBD of Atlantic cod and the Antarctic Notothenioids were activated by the ligands tested herein. In the in vitro assays with isolated liver cells of high-Antarctic Notothenioids, BaP exposure had no effect on overall respiration, but caused shifts in the respiration dedicated to protein synthesis. Thus, our study demonstrated that high-Antarctic fish possess a functional Ahr that can be ligand-activated in a concentration-dependent manner by environmental contaminants. This is associated with altered cost for cellular protein synthesis. Future studies have to show if the toxicant-induced activation of the Ahr pathway may lead to altered organism performance of Antarctic fish.
Supplementary information: The online version contains supplementary material available at 10.1007/s00300-021-02992-4.
Keywords: Aryl hydrocarbon receptor; Hepatocyte metabolism; Luciferase reporter gene assay; Notothenioids; Polycyclic aromatic hydrocarbons.
© The Author(s) 2021.
Figures

Similar articles
-
Persistent organic pollutants in red- and white-blooded High-Antarctic notothenioid fish from the remote Weddell Sea.Chemosphere. 2018 Feb;193:213-222. doi: 10.1016/j.chemosphere.2017.11.020. Epub 2017 Nov 7. Chemosphere. 2018. PMID: 29136567
-
Differential cellular metabolite alterations in HaCaT cells caused by exposure to the aryl hydrocarbon receptor-binding polycyclic aromatic hydrocarbons chrysene, benzo[a]pyrene and dibenzo[a,l]pyrene.Toxicol Rep. 2016 Sep 16;3:763-773. doi: 10.1016/j.toxrep.2016.09.003. eCollection 2016. Toxicol Rep. 2016. PMID: 28959603 Free PMC article.
-
Expression of aryl hydrocarbon receptor-regulated genes and superoxide dismutase in the Antarctic eelpout Pachycara brachycephalum exposed to benzo[a]pyrene.Environ Toxicol Chem. 2018 May;37(5):1487-1495. doi: 10.1002/etc.4075. Epub 2018 Feb 14. Environ Toxicol Chem. 2018. PMID: 29315775
-
Immunotoxicity of Xenobiotics in Fish: A Role for the Aryl Hydrocarbon Receptor (AhR)?Int J Mol Sci. 2021 Aug 31;22(17):9460. doi: 10.3390/ijms22179460. Int J Mol Sci. 2021. PMID: 34502366 Free PMC article. Review.
-
Biochemical adaptations of notothenioid fishes: comparisons between cold temperate South American and New Zealand species and Antarctic species.Comp Biochem Physiol A Mol Integr Physiol. 2007 Jul;147(3):799-807. doi: 10.1016/j.cbpa.2006.09.028. Epub 2006 Dec 5. Comp Biochem Physiol A Mol Integr Physiol. 2007. PMID: 17293146 Review.
References
-
- Aranguren-Abadía L, Lille-Langoy R, Madsen AK, Karchner SI, Franks DG, Yadetie F, Hahne ME, Goksoyr A, Karlsen OA. Molecular and functional properties of the atlantic cod (Gadus morhua) aryl hydrocarbon receptors Ahr1a and Ahr2a. Environ Sci Technol. 2020;54:1033–1044. doi: 10.1021/acs.est.9b05312. - DOI - PMC - PubMed
-
- Barnes DK, Peck LS. Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim Res. 2008;37:149–163.
LinkOut - more resources
Full Text Sources
