Luteal toxicity evaluation in rats
- PMID: 35221491
- PMCID: PMC8828616
- DOI: 10.1293/tox.2021-0058
Luteal toxicity evaluation in rats
Abstract
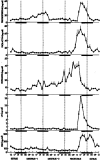
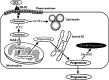
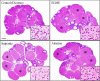
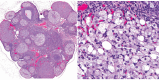
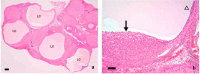
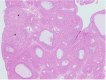
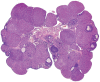
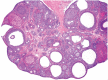
The corpora lutea (CL) are endocrine glands that form in the ovary after ovulation and secrete the steroid hormone, progesterone (P4). P4 plays a critical role in estrous and menstrual cycles, implantation, and pregnancy. The incomplete rodent estrous cycle stably lasts 4-5 days and its morphological features can be distinguished during each estrous cycle stage. In rat ovaries, there are two main types of CL: newly formed ones due to the current ovulation (new CL), and CL remaining from prior estrous cycles (old CL). In the luteal regression process, CL were almost fully regressed after four estrous cycles in Sprague-Dawley rats. P4 secretion from CL in rodents is regulated by the balance between synthesis and catabolism. In general, luteal toxicity should be evaluated by considering antemortem and postmortem data. Daily vaginal smear observations provided useful information on luteal toxicity. In histopathological examinations, not only the ovaries and CL but also other related tissues and organs including the uterus, vagina, mammary gland, and adrenal glands, must be carefully examined for exploring luteal changes. In this review, histological and functional characteristics of CL in rats are summarized, and representative luteal toxicity changes are presented for improved luteal toxicity evaluation in preclinical toxicity research.
Keywords: corpora lutea; luteal toxicity; ovary; progesterone; rat.
©2022 The Japanese Society of Toxicologic Pathology.
Figures



References
-
- Sanbuissho A, Yoshida M, Hisada S, Sagami F, Kudo S, Kumazawa T, Ube M, Komatsu S, and Ohno Y. Collaborative work on evaluation of ovarian toxicity by repeated-dose and fertility studies in female rats. J Toxicol Sci. 34(Suppl 1): SP1–SP22. 2009. - PubMed
-
- Stouffer RL. Structure, function, and regulation of the corpus luteum. In: Knobil and Neill’s Physiology of Reproduction, 3rd ed. JD Neill (ed). Elsevier Academic Press, San Diego. 475–526. 2006.
-
- Rothchild I. The regulation of the mammalian corpus luteum. Recent Prog Horm Res. 37: 183–298. 1981. - PubMed
-
- Yoshida M, Sanbuissyo A, Hisada S, Takahashi M, Ohno Y, and Nishikawa A. Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J Toxicol Sci. 34(Suppl 1): SP189–SP197. 2009. - PubMed
-
- Kaneko S, Sato N, Sato K, and Hashimoto I. Changes in plasma progesterone, estradiol, follicle-stimulating hormone and luteinizing hormone during diestrus and ovulation in rats with 5-day estrous cycles: effect of antibody against progesterone. Biol Reprod. 34: 488–494. 1986. - PubMed

