A Non-Interventional Multicenter Study of First-Line Bevacizumab in Combination with Chemotherapy in Patients with Metastatic Colorectal Cancer in Lebanon
- PMID: 35221671
- PMCID: PMC8881008
- DOI: 10.2147/BTT.S340525
A Non-Interventional Multicenter Study of First-Line Bevacizumab in Combination with Chemotherapy in Patients with Metastatic Colorectal Cancer in Lebanon
Abstract
Purpose: When combined with chemotherapy, bevacizumab improves progression-free survival (PFS) in metastatic colorectal cancer (mCRC). This observational trial was designed to assess the safety and efficacy of bevacizumab plus first-line chemotherapy in a real-world setting in Lebanon.
Patients and methods: A non-interventionaL multicenter study of first-LIne AVastin® (bevacizumab) in combination with chEmotherapy in patients with metastatic colorectal cancer (LLIVE) is a multicenter, prospective, Lebanon-based, observational study that enrolled mCRC patients who received first-line bevacizumab plus chemotherapy combination. The primary end point of the study was PFS. Secondary endpoints comprised the overall response rate (ORR) and the safety and tolerability of bevacizumab.
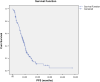
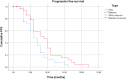
Results: A total of 196 patients were enrolled between July 2010 and August 2013. The median duration of follow-up was 11 months. Median duration of bevacizumab treatment was 4 months with FOLFOX being the chiefly chemotherapy regimen used in the first-line setting (26%). Median PFS was 8.22 months (95% confidence interval (CI): 7.005-9.443). The ORR was 50.3% (complete response 7.5%, partial response 42.8%). The most common adverse event encountered was hypertension (28%) followed by epistaxis (4.8%), diarrhea (4%), anemia (4%) and headache (4%). Grade 3/4 adverse events occurred in 15.2% of patients.
Conclusion: The trial further substantiated the efficacy and safety of bevacizumab and chemotherapy in the first-line treatment of mCRC patients in Lebanon.
Keywords: FOLFOX; XELOX; bevacizumab; metastatic colorectal cancer.
© 2022 Temraz et al.
Conflict of interest statement
Dr Layal Maatouk reports being an employee of Roche Lebanon SARL, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
References
-
- Incidence rates by site.MOPH; 2016. https://www.moph.gov.lb/en/Pages/8/19526/national-cancer-registry. Accessed November 29, 2021.
-
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760 - DOI - PMC - PubMed
-
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928 - DOI - PubMed
LinkOut - more resources
Full Text Sources