Integrated Disease Management for Chronic Obstructive Pulmonary Disease in Primary Care, from the Controlled Trial to Clinical Program: A Cohort Study
- PMID: 35221683
- PMCID: PMC8866979
- DOI: 10.2147/COPD.S338851
Integrated Disease Management for Chronic Obstructive Pulmonary Disease in Primary Care, from the Controlled Trial to Clinical Program: A Cohort Study
Abstract
Purpose: Integrated disease management (IDM) for COPD in primary care has been primarily investigated under clinical trial conditions. We previously published a randomized controlled trial (RCT) where the IDM intervention improved quality of life (QoL) and exacerbation-related outcomes. In this study, we assess the same IDM intervention in a real-world evaluation and identify patient characteristics associated with improved outcomes.
Methods: This historical cohort study included patients enrolled for 12 (±3 months) in the Best Care COPD IDM program. The main outcome was a ≥3 point improvement in COPD assessment test (CAT). Secondary outcomes were COPD exacerbations requiring antibiotics and/or prednisone, unscheduled physician visits, emergency department visits and hospitalizations.
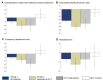
Results: Data for 571 patients (all patients) were included, 158 met the reference RCT eligibility (RCT matched). Improved QoL was observed in 43% (95% CI:38.9,47.2) of all patients, 47% (95% CI:39.5,55.6) of RCT matched vs 92% (95% CI:79.2,95.1) in the reference RCT intervention arm (n=72). Reductions (12 months IDM vs prior year) were observed in the proportion of patients experiencing exacerbation-related events (all patients): antibiotics/prednisone (-9.0%,95% CI:-13.9,-3.9); unscheduled physician (-33.1%,95% CI:-38.2,-27.9); emergency department (-9.6%,95% CI:-13.5,-5); and hospitalizations (-6.8%,95% CI:-10.0,-3.7). For the RCT matched group all reductions were comparable to the reference RCT intervention arm. The strongest predictors of improved QoL were baseline CAT, CAT≥20 vs CAT<10 (OR 15.6,95% CI:7.91,30.83), GOLD group B (OR 6.4,95% CI:3.42,11.85) and D (OR 5.64,95% CI:2.80,11.37) vs GOLD group A. Patients with prior antibiotic/prednisone use, FEV1 <30% predicted and GOLD group D were less likely to have no urgent health service utilization (OR 0.5,95% CI:0.30,0.68), (OR 0.2,95% CI:0.07,0.78) and (OR 0.3,95% CI:0.14,0.51), respectively.
Conclusion: Best Care COPD improved QoL and reduced exacerbation-related outcomes in a manner directionally similar to the RCT from which it emanated. Baseline QoL, exacerbation history, and GOLD category were identified as possible predictors of IDM impact and will inform future program development and resource allocation.
Keywords: COPD assessment test; chronic disease management; health service utilization; health status; quality of life.
© 2021 Hussey et al.
Conflict of interest statement
CL reports grants from Western University Professor of Health System Innovation; also reports personal fees for Advisory Board member for GlaxoSmithKline, AstraZeneca, Teva, Sanofi Genzyme and Valeo Pharma; and Research Grants from AstraZeneca; Honoraria or personal fees from AstraZeneca and GlaxoSmithKline, outside the submitted work. MF received honorarium from AstraZeneca outside the submitted work. The authors report no other conflicts of interests related to this study.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease. Report; 2022. Available from: https://goldcopd.org/wp-content/uploads/2021/11/GOLD-REPORT-2022-v1.0-12.... Accessed November, 2021.
-
- Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Can J Respir Crit Care Sleep Med. 2019;3(4):210–232. doi: 10.1080/24745332.2019.1668652 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

