Verification of the Usefulness of an Assessment and Risk Control Sheet that Promotes Management of Cancer Drug Therapy
- PMID: 35222016
- PMCID: PMC8864067
- DOI: 10.3389/fphar.2022.744916
Verification of the Usefulness of an Assessment and Risk Control Sheet that Promotes Management of Cancer Drug Therapy
Abstract
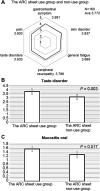
Background: Proper management of adverse events is crucial for the safe and effective implementation of anticancer drug treatment. Showa University Hospital uses our interview sheet (assessment and risk control [ARC] sheet) for the accurate evaluation of adverse events. On the day of anticancer drug treatment, a nurse conducts a face-to-face interview. As a feature of the ARC sheet, by separately describing the symptoms the day before treatment and the day of treatment and sharing the information on the medical record, it is possible to clearly determine the status of adverse events. In this study, we hypothesized that the usefulness and points for improvement of the ARC sheet would be clarified by using and evaluating a patient questionnaire. Methods: This study included 174 patients (144 at Showa University Hospital (Hatanodai Hospital) and 30 at Showa University Koto Toyosu Hospital (Toyosu Hospital) who underwent pre-examination interviews by nurses and received cancer chemotherapy at the outpatient center of Hatanodai and Toyosu Hospital. In the questionnaire survey, the ARC sheet's content and quality, respondents' satisfaction, structural strengths, and points for improvement were evaluated on a five-point scale. Results: The patient questionnaire received responses from 160 participants, including the ARC sheet use group (132 people) and the non-use group (28 people). Unlike the ARC sheet non-use group, the ARC sheet use group recognized that the sheet was useful to understand the adverse events of aphthous ulcers (p = 0.017) and dysgeusia (p = 0.006). In the satisfaction survey questionnaire, there was a high sense of security in the pre-examination interviews by nurses using the ARC sheet. Conclusions: The ARC sheet is considered an effective tool for comprehensively evaluating adverse events. Pre-examination interviews by nurses using ARC sheets accurately determined the adverse events experienced by patients with anxiety and tension due to confrontation with physicians.
Keywords: adverse event; assessment; common terminology criteria for adverse events; drug therapy; nursing; pre-examination.
Copyright © 2022 Honma, Watanabe, Fukuchimoto, Kashiwazaki, Tateba, Wagatsuma, Ogata, Maki, Sonou, Shiga, Otsuka, Hiruta, Hirasawa, Hosonuma, Murayama, Narikawa, Toyoda, Tsurui, Kuramasu, Kin, Kubota, Sambe, Horiike, Ishida, Shimada, Umeda, Tsunoda and Yoshimura.
Conflict of interest statement
Author MU was employed by the company Family Hospice Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Amézaga J., Alfaro B., Ríos Y., Larraioz A., Ugartemendia G., Urruticoechea A., et al. (2018). Assessing Taste and Smell Alterations in Cancer Patients Undergoing Chemotherapy According to Treatment. Support Care Cancer 26, 4077–4086. - PubMed
-
- Basch E., Iasonos A., McDonough T., Barz A., Culkin A., Kris M. G., et al. (2006). Patient versus Clinician Symptom Reporting Using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a Questionnaire-Based Study. Lancet Oncol. 7, 903–909. 10.1016/S1470-2045(06)70910-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

