Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease
- PMID: 35222026
- PMCID: PMC8867697
- DOI: 10.3389/fphar.2022.800950
Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease
Abstract
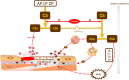
Chronic kidney disease (CKD) and cardiac insufficiency often co-exist, particularly in uremic patients on hemodialysis (HD). The occurrence of abnormal renal function in patients with cardiac insufficiency is often indicative of a poor prognosis. It has long been established that in patients with cardiac insufficiency, poorer renal function tends to indicate poorer cardiac mechanics, including left atrial reserve strain, left ventricular longitudinal strain, and right ventricular free wall strain (Unger et al., Eur J Heart Fail, 2016, 18(1), 103-12). Similarly, patients with chronic kidney disease, particularly uremic patients on HD, often have cardiovascular complications in addition to abnormal endothelial function with volume overload, persistent inflammatory states, calcium overload, and imbalances in redox responses. Cardiac insufficiency due to uremia is therefore mainly due to multifaceted non-specific pathological changes rather than pure renal insufficiency. Several studies have shown that the risk of adverse cardiovascular events is greatly increased and persistent in all patients treated with HD, especially in those who have just started HD treatment. Inflammation, as an important intersection between CKD and cardiovascular disease, is involved in the development of cardiovascular complications in patients with CKD and is indicative of prognosis (Chan et al., Eur Heart J, 2021, 42(13), 1244-1253). Therefore, only by understanding the mechanisms underlying the sequential development of inflammation in CKD patients and breaking the vicious circle between inflammation-mediated renal and cardiac insufficiency is it possible to improve the prognosis of patients with end-stage renal disease (ESRD). This review highlights the mechanisms of inflammation and the oxidative stress that co-exists with inflammation in uremic patients on dialysis, as well as the mechanisms of cardiovascular complications in the inflammatory state, and provides clinical recommendations for the anti-inflammatory treatment of cardiovascular complications in such patients.
Keywords: cardiovascular disease; chronic kidney disease; complement activation pathway; hemodialysis; immune response; inflammation; oxidative stress.
Copyright © 2022 Wang and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adda-Rezig H., Carron C., Pais de Barros J. P., Choubley H., Charron É., Rérole A. L., et al. (2021). New Insights on End-Stage Renal Disease and Healthy Individual Gut Bacterial Translocation: Different Carbon Composition of Lipopolysaccharides and Different Impact on Monocyte Inflammatory Response. Front. Immunol. 12, 658404. 10.3389/fimmu.2021.658404 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

