Ergothioneine Improves Aerobic Performance Without Any Negative Effect on Early Muscle Recovery Signaling in Response to Acute Exercise
- PMID: 35222093
- PMCID: PMC8864143
- DOI: 10.3389/fphys.2022.834597
Ergothioneine Improves Aerobic Performance Without Any Negative Effect on Early Muscle Recovery Signaling in Response to Acute Exercise
Abstract
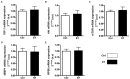
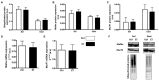
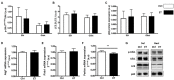
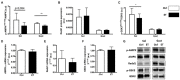
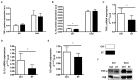
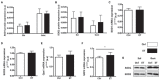
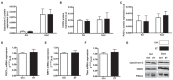
Physical activity is now recognized as an essential element of healthy lifestyles. However, intensive and repeated exercise practice produces a high level of stress that must be managed, particularly oxidative damage and inflammation. Many studies investigated the effect of antioxidants, but reported only few positive effects, or even muscle recovery impairment. Secondary antioxidants are frequently highlighted as a way to optimize these interactions. Ergothioneine is a potential nutritional supplement and a secondary antioxidant that activates the cellular NRF2 pathway, leading to antioxidant response gene activation. Here, we hypothesized that ergothioneine could improve performance during aerobic exercise up to exhaustion and reduce exercise-related stress without impairing early muscle recovery signaling. To test this hypothesis, 5-month-old C56B6J female mice were divided in two groups matched for maximal aerobic speed (MAS): control group (Ctrl; n = 9) and group supplemented with 70 mg ergothioneine/kg/day (ET; n = 9). After 1 week of supplementation (or not), mice performed a maximum time-to-exhaustion test by running on a treadmill at 70% of their MAS, and gastrocnemius and soleus muscles were collected 2 h after exercise. Time to exhaustion was longer in the ET than Ctrl group (+41.22%, p < 0.01). Two hours after exercise, the ET group showed higher activation of protein synthesis and satellite cells, despite their longer effort. Conversely, expression in muscles of metabolic stress and inflammation markers was decreased, as well as oxidative damage markers in the ET group. Moreover, ergothioneine did not seem to impair mitochondrial recovery. These results suggest an important effect of ergothioneine on time-to-exhaustion performance and improved muscle recovery after exercise.
Keywords: antioxidant; ergothioneine; exercise; exercise performance; exercise recovery; muscle.
Copyright © 2022 Fovet, Guilhot, Delobel, Chopard, Py and Brioche.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





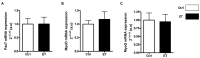
References
-
- Arc-Chagnaud C., Py G., Fovet T., Roumanille R., Demangel R., Pagano A. F., et al. . (2020). Evaluation of an antioxidant and anti-inflammatory cocktail against human Hypoactivity-induced skeletal muscle deconditioning. Front. Physiol. 11:71. doi: 10.3389/fphys.2020.00071, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

