Neuronal Population Transitions Across a Quiescent-to-Active Frontier and Bifurcation
- PMID: 35222095
- PMCID: PMC8867020
- DOI: 10.3389/fphys.2022.840546
Neuronal Population Transitions Across a Quiescent-to-Active Frontier and Bifurcation
Abstract
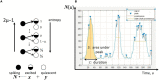
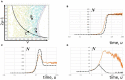
The mechanistic understanding of why neuronal population activity hovers on criticality remains unresolved despite the availability of experimental results. Without a coherent mathematical framework, the presence of power-law scaling is not straightforward to reconcile with findings implying epileptiform activity. Although multiple pictures have been proposed to relate the power-law scaling of avalanche statistics to phase transitions, the existence of a phase boundary in parameter space is until now an assumption. Herein, a framework based on differential inclusions, which departs from approaches constructed from differential equations, is shown to offer an adequate consolidation of evidences apparently connected to criticality and those linked to hyperexcitability. Through this framework, the phase boundary is elucidated in a parameter space spanned by variables representing levels of excitation and inhibition in a neuronal network. The interpretation of neuronal populations based on this approach offers insights on the role of pharmacological and endocrinal signaling in the homeostatic regulation of neuronal population activity.
Keywords: differential inclusion; epileptiform activity; excitation-inhibition balance; homeostatic regulation; neuronal avalanches.
Copyright © 2022 Juanico.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Deviations from Critical Dynamics in Interictal Epileptiform Activity.J Neurosci. 2016 Nov 30;36(48):12276-12292. doi: 10.1523/JNEUROSCI.0809-16.2016. J Neurosci. 2016. PMID: 27903734 Free PMC article.
-
Balance of excitation and inhibition determines 1/f power spectrum in neuronal networks.Chaos. 2017 Apr;27(4):047402. doi: 10.1063/1.4979043. Chaos. 2017. PMID: 28456161
-
Scale free avalanches in excitatory-inhibitory populations of spiking neurons with conductance based synaptic currents.J Comput Neurosci. 2023 Feb;51(1):149-172. doi: 10.1007/s10827-022-00838-4. Epub 2022 Oct 25. J Comput Neurosci. 2023. PMID: 36280652 Free PMC article.
-
Scaling of avalanche shape and activity power spectrum in neuronal networks.Phys Rev E. 2022 Aug;106(2-1):024304. doi: 10.1103/PhysRevE.106.024304. Phys Rev E. 2022. PMID: 36109993
-
Statistical analyses support power law distributions found in neuronal avalanches.PLoS One. 2011;6(5):e19779. doi: 10.1371/journal.pone.0019779. Epub 2011 May 26. PLoS One. 2011. PMID: 21720544 Free PMC article.
References
-
- Benaïm M., Hofbauer J., Sorin S. (2005). Stochastic approximations and differential inclusions. SIAM J. Control Optimiz. 44 328–348.
-
- Costa A. A., Brochini L., Kinouchi O. (2017). Self-organized supercriticality and oscillations in networks of stochastic spiking neurons. Entropy 19:399.
LinkOut - more resources
Full Text Sources

