The Regulation of Integrated Stress Response Signaling Pathway on Viral Infection and Viral Antagonism
- PMID: 35222313
- PMCID: PMC8874136
- DOI: 10.3389/fmicb.2021.814635
The Regulation of Integrated Stress Response Signaling Pathway on Viral Infection and Viral Antagonism
Abstract
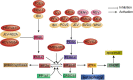
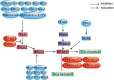
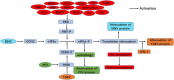
The integrated stress response (ISR) is an adaptational signaling pathway induced in response to different stimuli, such as accumulation of unfolded and misfolded proteins, hypoxia, amino acid deprivation, viral infection, and ultraviolet light. It has been known that viral infection can activate the ISR, but the role of the ISR during viral infection is still unclear. In some cases, the ISR is a protective mechanism of host cells against viral infection, while viruses may hijack the ISR for facilitating their replication. This review highlighted recent advances on the induction of the ISR upon viral infection and the downstream responses, such as autophagy, apoptosis, formation of stress granules, and innate immunity response. We then discussed the molecular mechanism of the ISR regulating viral replication and how viruses antagonize this cellular stress response resulting from the ISR.
Keywords: eIF2α phosphorylation; host; integrated stress response; unfolded protein response; viral replication.
Copyright © 2022 Wu, Zhang, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

