Precision Vaccine Development: Cues From Natural Immunity
- PMID: 35222350
- PMCID: PMC8866702
- DOI: 10.3389/fimmu.2021.662218
Precision Vaccine Development: Cues From Natural Immunity
Abstract
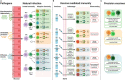
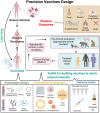
Traditional vaccine development against infectious diseases has been guided by the overarching aim to generate efficacious vaccines normally indicated by an antibody and/or cellular response that correlates with protection. However, this approach has been shown to be only a partially effective measure, since vaccine- and pathogen-specific immunity may not perfectly overlap. Thus, some vaccine development strategies, normally focused on targeted generation of both antigen specific antibody and T cell responses, resulting in a long-lived heterogenous and stable pool of memory lymphocytes, may benefit from better mimicking the immune response of a natural infection. However, challenges to achieving this goal remain unattended, due to gaps in our understanding of human immunity and full elucidation of infectious pathogenesis. In this review, we describe recent advances in the development of effective vaccines, focusing on how understanding the differences in the immunizing and non-immunizing immune responses to natural infections and corresponding shifts in immune ontogeny are crucial to inform the next generation of infectious disease vaccines.
Keywords: adaptive immunity; adjuvants; antigens; immune system; innate immunity; natural infection; vaccines; vita-PAMP.
Copyright © 2022 Barman, Soni, Brook, Nanishi and Dowling.
Conflict of interest statement
DD is a named inventor on granted patents and patent applications related to vaccine adjuvants and vaccine formulation design. DS, BB and EN are named inventor on multiple patent applications focused on the design of the adjuvanted vaccine formulation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

